Heart failure is one of the commonest and most costly causes for hospital admission in both Europe and the United States (US). Eleven percent of patients who present with acute heart failure die in hospital followed by an overall one year mortality of 30%1. Re-admission rates are 20% at four weeks and 50% by six months. Substantial efforts are being made to lessen the symptomatic and economic burden of these high-risk patients; however, this is a difficult population to address. From the Nationwide Inpatient Sample (US), 53% of admissions are in patients older than 75 years, often characterised by important comorbidities2. Seventy percent are hypertensive, 42% are in atrial fibrillation, 41% suffer diabetes, 40% have renal impairment, 38% suffer mental illness, 30% have chronic obstructive pulmonary disease and 30% are anaemic2. The clinical course of chronic heart failure is difficult to predict and very few patients have access to anything other than palliative care (Figure 1)3.
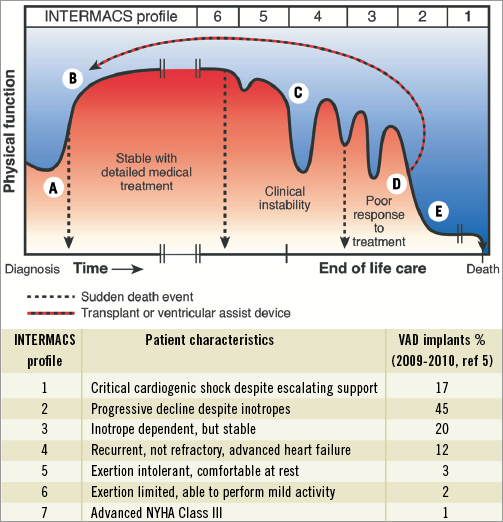
Figure 1. The unpredictable course of chronic heart failure. A) Symptomatic onset, hospitalisation, diagnosis and initiation of medical treatment. Some (around 11%) die at this stage. B) Stable on drugs, + cardiac resynchronisation therapy. Exercise limitation and risk of sudden death persist (NYHA II-III). C) Periods of instability related to dysrhythmia or renal dysfunction. Worsening exercise tolerance (NYHA III-IV). May receive implantable cardiodefibrillator. D) Poor response to treatment (NYHA IV) requiring multiple hospital admissions. High risk of decompensations and sudden death. E) Terminal phase, often with cardiogenic shock and multiple organ failure. Hospitalised on inotropes or an intra-aortic balloon pump. Modified from Goodlin SJ3 (with permission).
Given the limits of medical treatment and cardiac resynchronisation therapy no aspect of cardiology has witnessed a more dramatic growth than the use of left ventricular assist devices (LVAD). Unlike cardiac transplantation where worldwide numbers have plateaued at 4,500 operations annually, the US alone predicts implantation of between 150,000 to 250,000 LVADs per year4. These estimates are based on existing selection criteria in severely symptomatic heart failure patients (INTERMACS 1-3) who are hospitalised and dependent upon inotropic support for survival (Figure 1)5. Only 18% of long-term LVAD patients are implanted before the need for inotropic support5. For the pre-terminal patient, outcome is critically dependent upon end-organ dysfunction and the ability to survive an invasive surgical procedure6. To withhold treatment until cardiogenic shock with established multi-organ failure places both the patient’s life and the $100,000 cost of the device in jeopardy.
As the durability and safety of implantable blood pumps increases, there is compelling evidence to deploy them in less sick patients on an elective basis7,8. This strategy has already improved both hospital and long-term survival whilst the LVAD provides unparalled symptomatic relief, better quality of life and improved longevity for the majority of patients9. Unlike transplantation, there is no restriction on availability or age limit for LVADs. They can be used successfully in the presence of pulmonary hypertension, renal impairment and other comorbidities which preclude transplantation10. As experience extends into the elderly population, LVADs are implanted through smaller incisions without cardiopulmonary bypass or with minimal extracorporeal circulation (MECC)8,11.
In this journal Yi and colleagues report on an impressive “proof of concept” animal study which examines the potential for minimally invasive implantation of the extra-thoracic CircuLite® SYNERGY® pocket Micro-pump (CircuLite®, Inc., Teaneck, NJ, USA)12. First used clinically in 2007, this small (25 g, 49 mm×14 mm) pump was designed to provide partial circulatory support (around 3 litres/minute in vivo) and relieve symptoms in patients who are not yet sick enough for a conventional implanted LVAD (NYHA III/IV, INTERMACS 4-7)13. These patients are otherwise served by drug and cardiac resynchronisation therapy, but remain limited functionally as well as by heart-failure-related comorbidity. The objective of making these patients more comfortable and avoiding hospital admissions clearly has merit. In the current clinical configuration, a 20.5 cm length, 6 mm diameter nitinol reinforced silicone inflow cannula is implanted into the left atrium via a limited right thoracotomy14. The proximal end exits the thorax through the second intercostal space adjacent to a 4 cm right subclavicular incision used to expose the right subclavian artery. A subcutaneous device pocket (akin to a pacemaker pocket) is created anterior to the right pectoral muscle. The power cable is tunnelled caudally to exit the right upper quadrant and an 8 mm PTFE outflow graft conveys pump flow to the subclavian artery. The impeller spins at between 20,000 and 28,000 rpm and depends upon afterload (the lower the blood pressure the greater the flow). The patient is prescribed aspirin 100 mg per day and anticoagulated with warfarin to achieve an INR between 2.5-3.0.
With the modifications described by Yi et al, percutaneous insertion of the inflow cannula via the right jugular vein and superior vena cava is followed by transseptal puncture. As a result, this innovative device may well enter the domain of the interventional cardiologist12. Whilst this is an appealing concept, miniaturisation comes at a price. The long inflow cannula from the low pressure left atrium might be expected to predispose to device thrombosis. This is why the Jarvik Flowmaker (Jarvik Heart Inc., New York, NY, USA) and HeartWare (HeartWare Inc., Framingham, MA, USA) LVADs were specifically designed to avoid an inflow cannula by inserting the pump itself directly into the left ventricular apex10. Also, with a pump rotor speed exceeding 20,000 rpm, high sheer stress denatures von Willebrand factor, thereby adding an unintentional factor deficiency to the anticoagulant regime and predisposing to bleeding15. Other LVADs also do this.
The reported CircuLite® SYNERGY® clinical experience is predominantly in INTERMACS profile 4 patients (non-inotrope dependent UNOS Status II) in a bridge to transplant setting. This is a limited population since only UNOS Status I patients are shown to derive survival benefit from transplantation9. In the first reported clinical series (2009), Meyns et al confirmed an appropriate increase in cardiac index with reduction in capillary wedge pressure, but eight of the first 12 pumps thrombosed leading to structural modifications within the device14,16. In a second clinical report, Meyns et al described 27 bridge-to-transplant patients where the duration of support ranged from six to 281 days17. Again, the INTERMACS profile 4 patients showed significant haemodynamic and symptomatic improvement, but pump thrombosis was not included in the table of adverse events. The authors noted that severe adverse events after initial recovery (first 30 days) occurred at a higher rate compared with the HeartMate II (Thoratec Corporation, Pleasanton, CA, USA) LVAD (5.9 events per patient year versus 3.8 events per patient year respectively), but the invited discussant (European Society of Cardiothoracic Surgery meeting 2010) indicated that the pump thrombosis rate approached 50%. Also the few INTERMACS profile 2 and 3 patients did not achieve the treatment goals of withdrawal from inotropic support or hospital discharge. Again, further modifications have been made to the pump in an attempt to reduce propensity for thrombosis.
How important is a minimally invasive LVAD implantation?
In conventional cardiac surgery, median sternotomy and cardiopulmonary bypass are used routinely for the elderly (80-90 years) heart failure patients undergoing coronary bypass or valve replacement surgery. Equally, experience with standard LVAD implantation shows similar outcomes in the elderly as for younger patients8,11. For those with significant comorbidity, it is more important to avoid debilitating complications and multiple interventions. Whilst the CircuLite® SYNERGY pump is clearly innovative and well intentioned, it is still not clear which patients would achieve significant benefit. The pump is not powerful enough for salvage from cardiogenic shock or bridge to transplant in INTERMACS profiles 1-3 (UNOS Status I)17. It could be made substantially larger and still be deployed less invasively. Over six years of reported clinical experience, the longest pump run was 281 days, which is insufficient for CircuLite® to be considered a destination therapy device14,16,17. At the same time, increased safety and durability of the long-term rotary blood pumps have already encouraged their use in the population which CircuLite® intended to address18. As a result, there are practical issues to address before the target population is defined and the principle can be supported by an appropriate evidence base.
The fact that cardiologists may be able to implant CircuLite is not enough. As for TAVI, the durability and complication rates must approximate to those of conventional LVADs before the less invasive concept is widely accepted.
Conflict of interest statement
S. Westaby is Consultant Cardiac Surgeon, Oxford University Hospitals Trust and also Medical Director (unpaid) of Calon Cardiotechnology, the blood pump bioengineering group at the Institute of Life Sciences, University of Swansea, Wales, United Kingdom.

