Evidence-based medicine aims to overcome eminence-based decision making in clinical practice. Cardiology can take pride in being at the forefront of this development; no specialty has witnessed a similar abundance of diagnostic and therapeutic advances related to imaging, biomarkers, prevention, drugs, devices and minimally invasive interventions with profound impact on quality of life and life expectancy. Clinical practice guidelines issued by the European Society of Cardiology (ESC) and the American College of Cardiology/American Heart Association (ACC/AHA) regularly integrate novel data into recommendations classified by class and level of evidence (LOE). Whilst only a small overall fraction of recommendations obtain the highest level of evidence (LOE A) reflecting the continued unmet need for more randomised clinical trials, the contribution of interventional cardiology to the evidence base in the specialty of cardiology during the past four decades has been remarkable1.
Several observations pertain to the prolific generation of novel evidence by interventional cardiology to the field of cardiovascular medicine at large:
– The key mission of the specialty is to provide minimally invasive rather than open surgical correction of cardiac diseases. The associated benefits in terms of prompt recovery and restoration of quality of life as well as cost saving are intuitively attractive to patients, healthcare organisations and payers. Transcatheter aortic valve implantation (TAVI) is a recent example, demonstrating efficacy comparable to surgical aortic valve replacement (SAVR) while providing more rapid restoration of quality of life and less intense health resource utilisation such as hospital stay, need for rehabilitation and overall cost2. The amount of evidence in terms of randomised clinical trials published during only one decade in a field devoid of randomised evidence prior to the advent of TAVI is truly impressive and has impacted on guidelines3.
– The key driver for the generation of new evidence is innovation provided by the fruitful interaction of interventional cardiologists and device engineers identifying unmet needs in search of therapeutic solutions. The list of “first” therapeutic interventions in the history of interventional cardiology is long (Figure 1), and is expected to expand further in the future. It seems that there are no limits to the ingenuity of both physicians and engineers to push the boundaries which in turn give impetus to prospective evaluation of new technologies in clinical trials. As the life cycle of devices is typically shorter than that of drugs, thereby intrinsically accelerating the innovation cycle, any device iteration itself triggers novel studies and related evidence.
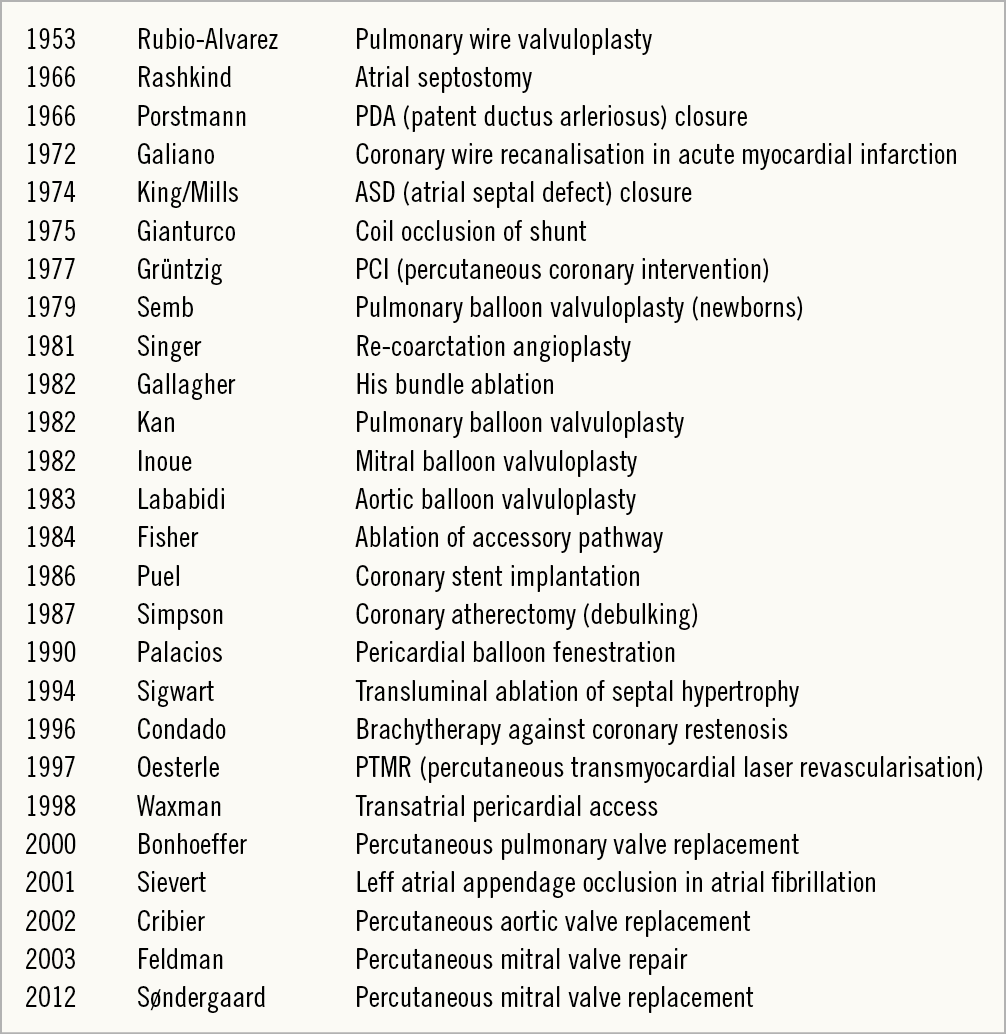
Figure 1. Historical overview of innovative therapeutic contributions in invasive cardiology. Courtesy of Bernhard Meier.
– The key vision of interventional cardiology as a specialty is its collaborative spirit, particularly when engaging cardiac surgeons and imaging specialists. The concept of the Heart Team was introduced in the 2010 joint ESC/EACTS guidelines on myocardial revascularisation and has not only been adopted universally but also extended to other fields, including valvular heart disease, heart failure, and atrial fibrillation4. The interaction between various specialties proved to be fertile not least due to the competition propelling the field forward. This is exemplified by the number of randomised clinical trials comparing percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG), establishing revascularisation as one of the best studied therapeutic interventions in medicine (Figure 2). The most recent edition of the joint ESC/EACTS guidelines on myocardial revascularisation once more emphasises the importance of the Heart Team while providing practical guidance on how to guide clinical decision making in individual patients with multivessel coronary artery disease, taking into consideration disease extent as assessed by the SYNTAX score, the presence of left main disease as well as diabetic status5,6.
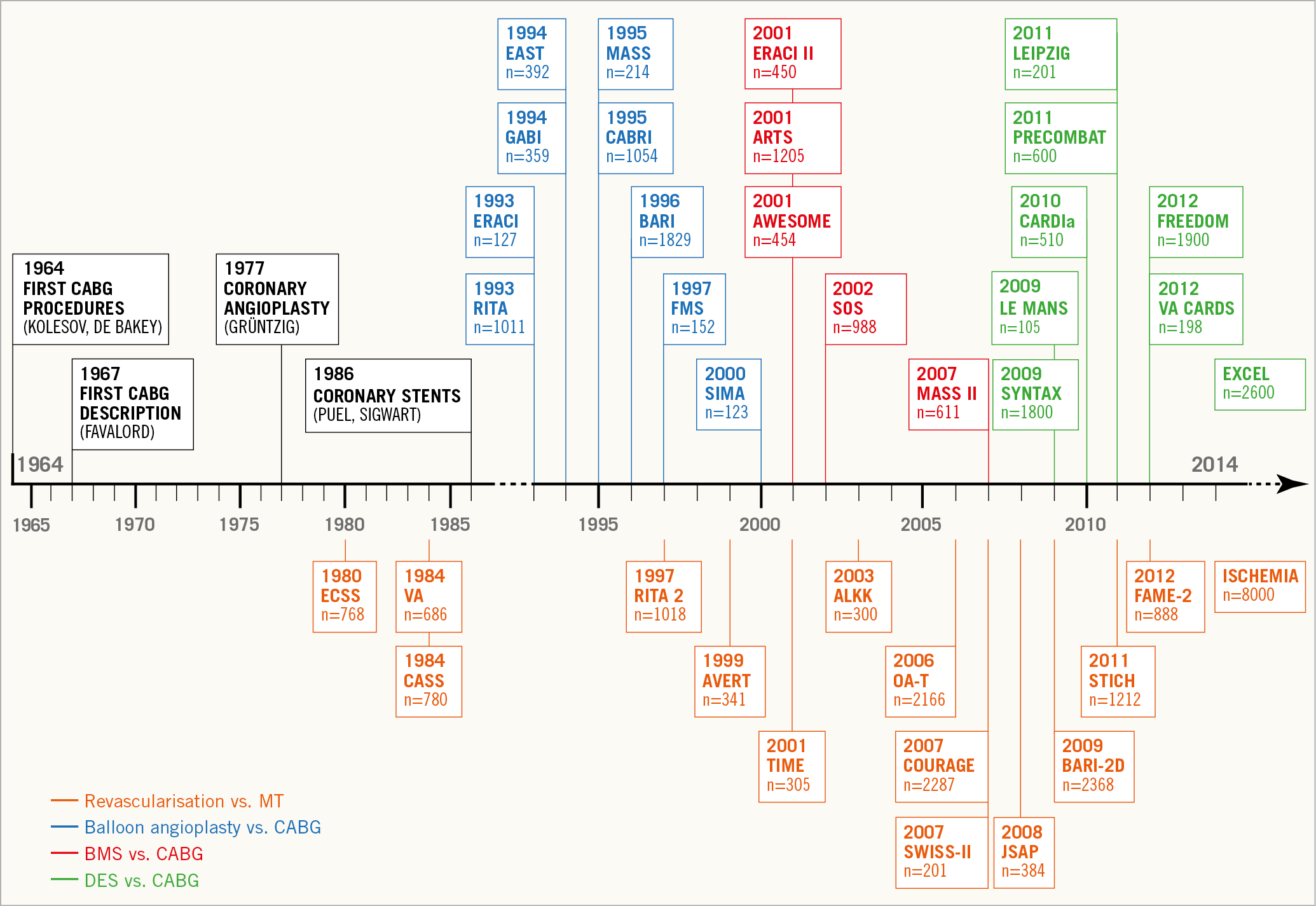
Figure 2. Randomised clinical trials comparing percutaneous coronary intervention and coronary artery bypass surgery in patients with coronary artery disease. Reproduced with permission from Windecker et al6.
– The key asset of interventional cardiology is the seamless symbiosis of device and drug innovation. Indeed, remarkable progress in adjunct pharmacology decisively contributed to the progress in the field of interventional cardiology. The shift from oral anticoagulation to dual antiplatelet therapy as adjunct antithrombotic treatment among patients undergoing coronary stent implantation was pioneered by interventional cardiologists in the early 1990s. The routine use of dual antiplatelet therapy for PCI not only improved the safety and efficacy of the procedure but also resulted in the development of novel P2Y12 inhibitors with more potent and reversible platelet inhibition features and unprecedented generation of evidence in randomised clinical trials (Figure 3),7. Similarly, the advent of non-vitamin K oral anticoagulants (NOAC) triggered a number of trials in the specific setting of atrial fibrillation patients presenting with ACS and/or undergoing PCI. Indeed, the abundant evidence and proliferative research in this field have led to a subspecialty referred to as thrombocardiology dedicated to studying the delicate balance between ischaemic protection and bleeding prevention8.
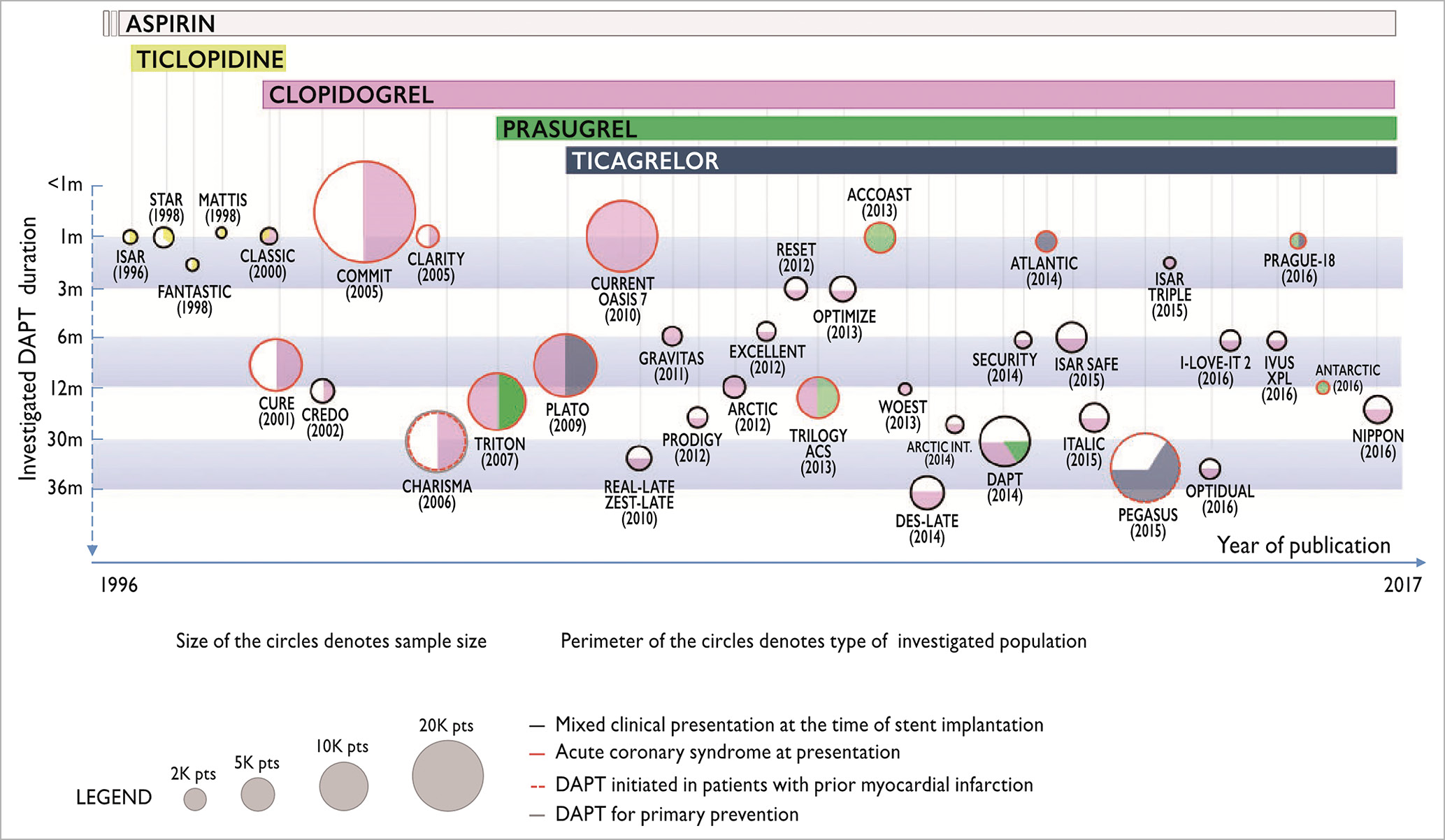
Figure 3. Randomised clinical trials investigating the safety and efficacy of dual antiplatelet therapy in patients with coronary artery disease. Reproduced with permission from Windecker et al6.
The future of interventional cardiology will continue to be bright and will be dictated by the progress ahead of us. The ever accelerating knowledge cycle, advances in artificial intelligence, 3D printing, novel materials, innovative drug designs and precision medicine will fuel the engine of innovation. As a result, clinical trials will subtend new device and drug innovations to the reality of clinical practice and inform the community of the best available evidence. Collectively, these results will hopefully increase the proportion of recommendations with the highest level of evidence to the benefit of patients and healthcare providers in the not so distant future.
Conflict of interest statement
S. Windecker reports research and educational grants to the institution from Abbott, Amgen, Bayer, BMS, Boston Scientific, Biotronik, Medtronic, Edwards Lifesciences, Sionmed and Polares.

