Abstract
With the rapid expansion of interventional therapies for structural heart disease, it is no surprise that the field of interventional heart failure (HF) is now an established reality. Why is there a need for interventional treatment of HF? Despite critical advances in the treatment of some forms of HF, there are still major unmet needs in the HF field (e.g., HF with preserved ejection fraction and right ventricular failure), and HF-related morbidity and mortality remain high. Furthermore, there are several advantages to device-based therapies for HF: they may help reduce polypharmacy and the need for patient compliance with pharmacotherapies, both of which continue to plague the treatment of HF. For these reasons and others, there has been a plethora of development within the interventional HF field, with therapies ranging from interatrial shunt devices to left ventricular partition devices. Here we discuss the current unmet need for interventional HF therapies, lessons learned from prior successes and challenges in the development of device-based HF therapeutics, novel interventional therapies on the horizon for HF patients, and future challenges that will be critical for all those in the field to consider when developing interventional therapies for HF.
Introduction
THE UNMET NEED FOR EFFECTIVE DEVICE-BASED THERAPEUTICS FOR HEART FAILURE
Despite major advances in the care of heart failure (HF), particularly in chronic HF with reduced ejection fraction (HFrEF), morbidity and mortality for HF remain high. Furthermore, the prevalence of HF, particularly HF with preserved EF (HFpEF), continues to increase, a trend that is expected to continue in the coming decades given the ageing of the population and the rising prevalence of obesity, diabetes, and hypertension1. Concurrently, there has been a veritable explosion in the development of interventional therapies for structural heart disease, including HF – a natural extension of the success of interventional cardiology and the ongoing unmet need for additional therapies for HF.
Why is there a need for interventional HF therapies? In addition to the aforementioned persistently high morbidity and mortality of HF patients, there are some specific aspects within the HF field that make interventional therapies attractive. First, polypharmacy and medication non-compliance are significant problems in patients with HF. A major advantage of many device-based therapies is the ability to treat the patient without the need to add to an already high number of medications that require patient compliance and can interact with each other, resulting in adverse events. Second, in patients with chronic HFrEF, further development of neurohormonal therapies is severely limited due to lack of room for additional blood pressure lowering. Finally, several HF syndromes – including acute decompensated HF, right ventricular failure, and HFpEF – have proven to be quite resistant to improvement with pharmacologic approaches. Thus, the possibility of interventional therapies for HF is quite exciting and could fill large gaps in the treatment of HF.
INTERVENTIONAL TREATMENT OF HEART FAILURE: DESTINED TO THRIVE BASED ON PRIOR SUCCESSES
Although interventional treatment of HF is a relatively new concept and one that can be considered a “new field”, in reality there have been several prior examples of successful interventional devices for HF. Prior “interventional” therapies that have improved outcomes in HF include cardiac resynchronisation therapy (CRT)2 and invasive haemodynamic monitoring (e.g., CardioMEMS™ HF System; St. Jude Medical, St. Paul, MN, USA)3. Even atrial septostomy, a palliative procedure for patients with pulmonary arterial hypertension (PAH) with severe right heart failure, has been shown to improve symptoms by increasing left heart cardiac output in severely ill PAH patients4. Similarly, short-term mechanical circulatory support devices, implanted percutaneously, have provided temporary assistance for severely ill HF patients who require a bridge to recovery from an acute illness or a more permanent therapeutic solution5.
What can we learn from these prior successes? In the case of CRT, determining which HF patients will benefit most from the device has been a vexing question, and one that continues to evolve. What is clear is that there is a subgroup of HF patients (e.g., those with reduced EF, non-ischaemic cardiomyopathy, wide QRS >150 ms2, and possibly a left bundle branch block QRS pattern) who seem to benefit most from CRT therapy. However, it took several trials and considerable clinical experience to determine the “CRT responder” phenotype, and there is still debate as to which patients will do best with CRT, and the best metric to determine a beneficial “response”.
In the case of CardioMEMS, the CHAMPION randomised controlled trial showed that invasive haemodynamic monitoring significantly decreased HF hospitalisation in patients with HF (regardless of underlying EF)3. Despite these positive results, the US Food and Drug Administration (FDA) had concerns about statistical analysis techniques and also e-mail communications from the sponsor and principal investigators to the sites, with potentially unbalanced recommendations for patients in the control vs. intervention groups. While further analysis of the data and communication with the FDA alleviated these concerns and ultimately led to approval of CardioMEMS, these problems highlight the unique challenges of designing and conducting device-based trials in HF.
What can we learn from the CRT and CardioMEMS experiences? HF, by definition, is a heterogeneous syndrome. Thus, disease mechanisms are likely to vary considerably among patients, and appropriate “matchmaking” of therapies, HF patient subtypes, and clinical trial outcomes is critical6. However, predicting the subtype of HF that will respond best to a particular therapy can be difficult; therefore, the design of initial clinical trials and novel post hoc analyses of these trials can be helpful. Deep phenotyping (i.e., gathering quantitative data from multiple domains in patients enrolled in clinical trials) along with machine learning to determine which combination of these phenotypes best predicts patients who respond best to a particular therapy (i.e., supervised statistical learning analyses of responders) is a novel method to speed up the development process by identifying the most appropriate responders who should be enrolled in subsequent trials7. Such an approach in the early CRT trials may have identified the characteristics of “responders” outlined above much sooner and at a much lower cost than the several subsequent CRT trials that were conducted.
Although the challenges of the CardioMEMS trial were different from those for CRT, the solution is within the same realm - careful consideration of trial design in the planning phases prior to study enrolment. The CardioMEMS experience teaches us that issues of blinding are some of the most difficult for device-based trials, and every precaution must be considered when designing device trials to avoid future negative scrutiny or potential invalidation of trial results upon completion of the trial.
NOVEL INTERVENTIONAL THERAPEUTICS FOR HEART FAILURE
Figure 1 displays examples of existing and novel interventional therapies for HF. There are several novel HF devices currently in development. In general, these devices fall into one of several categories: (1) devices to diagnose worsening HF (invasive haemodynamic monitors); (2) devices that leverage cardiac anatomy in some way to achieve their goals (interatrial shunt devices, left ventricular [LV] elastic expanders that transfer energy from systole to diastole, and LV partitioning devices to decrease LV wall stress); (3) devices such as carotid baroreceptor stimulators (and CRT) that take advantage of electrical properties of the cardiovascular system; and (4) cardiac assist devices which in the future may be helpful beyond acute decompensated HF (e.g., CircuLite® Synergy® system [HeartWare, Framingham, MA, USA] may be a novel treatment option for HFpEF8). While the interventional HF therapies currently in development have yet to be proven beneficial, they have clearly benefited from advances in engineering and material sciences coupled with clinical ingenuity, and demonstrate the overall potential of the field.
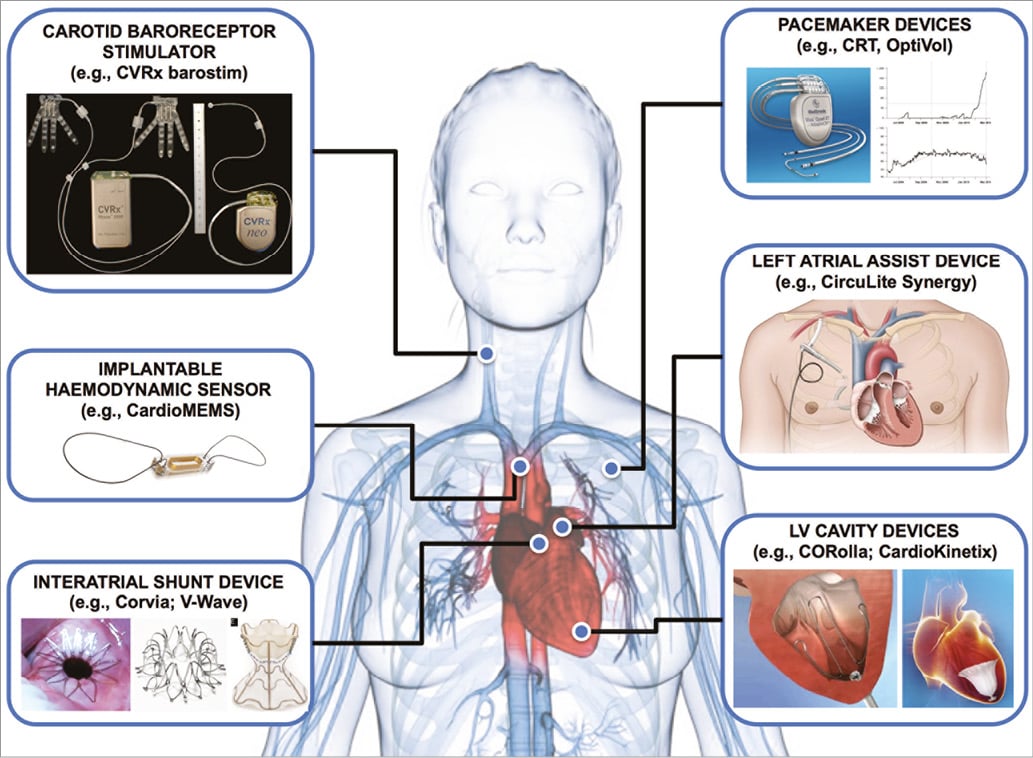
Figure 1. Examples of interventional therapies for heart failure. Several interventional therapies for heart failure have been developed in the past or are currently in development.
Future challenges
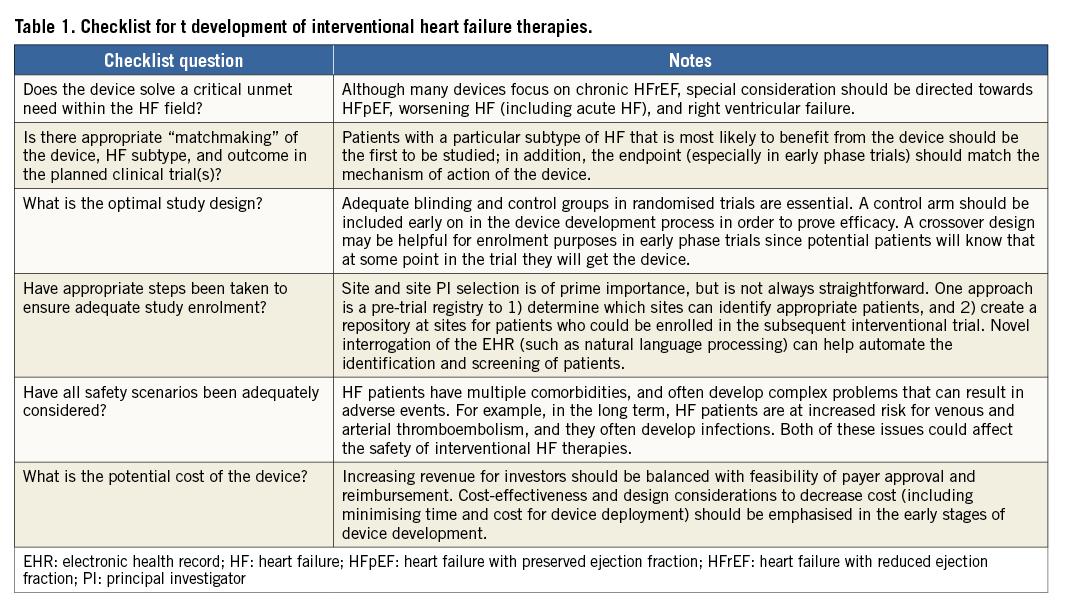
Although there have been prior successes in the realm of interventional therapy for patients with HF, there have also been important lessons learned from prior device trials such as those outlined above. Each of these lessons recounts challenges that should be considered when developing novel intervention-based therapeutics. Table 1 provides a suggested checklist for all those involved in the development of devices for patients with HF. At each phase of development, it is important for inventors, investigators, interventional cardiologists, HF specialists, industry representatives, regulators, and payers to think clearly about the rationale (i.e., unmet need), potential benefits, potential markets, and costs for any new interventional therapeutic device.
Conclusions
Based on prior success in HF and the ever-expanding use of structural heart disease interventions in valvular heart disease and congenital heart disease, the future is bright for the new field of interventional HF. Nonetheless, the development of interventional HF therapies will benefit from understanding the most pressing unmet needs in HF, lessons learned from prior HF clinical trials of device-based therapies (particularly the importance of optimal study designs and matchmaking of HF subtype to appropriate therapies), and the need to balance cost-effectiveness and cost containment with continued industry investment in the development of novel interventional HF therapeutics.
Conflict of interest statement
S. Shah has received research funding from the National Institutes of Health (R01 HL107577, R01 HL127028), American Heart Association (16SFRN28780016), Novartis, and Actelion, and consultant fees from Bayer, Merck, and Novartis. S. Shah is also a principal investigator of the REDUCE LAP-HF I clinical trial, which is sponsored by Corvia Medical, Inc.

