Abstract
Patients with elevated filling pressures are at increased risk of adverse cardiovascular (CV) outcomes. Structural interventions to lower elevated either left or right atrial filling pressures are gaining attention. Studies in heart failure show that lowering left atrial pressure may reduce CV events while improving functional capacity. In recognition of this, trials are ongoing studying the effects of percutaneously implanted interatrial shunt devices (IASD). The preliminary results of IASD implantation suggest that periprocedural complications are rare and midterm safety good. Although both haemodynamic and functional parameters improve after IASD implantation, study designs, including sample size and duration, preclude definite conclusions regarding potential efficacy. In this paper, we briefly summarise current knowledge in the field, and give a perspective on the data needed to make interatrial device shunt therapy a part of our armamentarium in patients with heart failure or pulmonary hypertension and increased filling pressure.
Introduction
The observation that patients with mitral stenosis and congenital atrial septal defect (ASD) were less symptomatic than patients with mitral stenosis and no ASD led Dr Lutembacher in 1916 to suggest that atrial septal defects may have beneficial haemodynamic effects in patients with increased left atrial pressures. As the degree of increased filling pressures in heart failure (HF) is associated with symptom severity and mortality1 and decreasing filling pressures may improve outcomes2 there is an incentive to explore iatrogenic ASD as a treatment option. Given the absence of effective treatment options especially for heart failure with preserved ejection fraction (HFpEF), Lutembacher’s early observation has sparked a new line of trials. The concept of iatrogenic ASD has also been explored in patients with severe pulmonary hypertension.
Therapeutic left-to-right shunt
Several studies have indicated that left atrial pressure is a key parameter in determining symptoms and outcomes in left-sided HF. Earlier studies have shown that there is an association between left ventricular (LV) filling pressure and death and HF hospitalisations. In a retrospective study, Dorfs and co-workers showed that every 1 mmHg increase in pulmonary capillary wedge pressure was associated with a significant 9% (2-11%) increase in mortality1. Furthermore, a pilot study using an implantable left atrial pressure (LAP) sensor to guide daily treatment of HF patients (n=40) showed that intensified medication led to a drop of 2.8 mmHg in LAP pressure2. This translated into a 59% (p=0.04) decrease in the combined endpoint of mortality and HF hospitalisation. These results support the concept of reducing LA filling pressures in HF. Given the compliance of the right side of the heart, one way to decrease LA filling pressure is to create an atrial shunt allowing near equalisation of atrial pressures.
Catheter-guided atrial transseptal puncture is the simplest means of creating an interatrial shunt. Transseptal puncture is commonly performed as part of several cardiac interventions. In fact, there are indications that transseptal catheter placement may explain some of the symptom relief that catheter-guided mitral valve interventions provide in patients with HF and mitral regurgitation. Using a 22 Fr guiding catheter routinely used for MitraClip® (Abbott Vascular, Santa Clara, CA, USA) procedures in 28 patients, an averaged 0.19 cm2 atrial septal defect was observed using 3D echocardiography3. It is unclear whether such a procedure produces a long-term patent shunt. Furthermore, the size of the ASD created is unpredictable and in the abovementioned study the septal defects ranged from 0.12-0.28 cm2. To counter these problems, percutaneous interatrial shunt devices with well-defined opening areas are currently being investigated. One major question is the optimal size of a therapeutic ASD. This has been addressed using mathematical modelling based on human haemodynamic data from HFpEF patients, yielding an optimum of 8-9 mm (~0.5-0.6 cm2)4. A balance exists between the minimum required reduction in filling pressure and increases in shunt diameter that progressively lower filling pressure while attenuating LV cardiac output and straining the right ventricle (RV). However, a diameter of 8-9 mm is well below the size believed to induce secondary pulmonary hypertension caused by iatrogenic left-to-right shunting.
There are currently two interatrial shunt devices that have been evaluated in early trials (IASD®; Corvia Medical Inc., Tewksbury, MA, USA and V-Wave®; V-Wave Ltd., Caesarea, Israel) (Figure 1). Treated patients differ in that the IASD device trials have only included HFpEF patients, whereas the V-Wave device has been applied both to patients with HF and reduced ejection fraction (HFrEF) and to those with HFpEF. The common denominator is that selection criteria for both devices included augmented left atrial filling pressures. The Corvia IASD is a simple nitinol frame creating an 8 mm opening when expanded, whereas the V-Wave has a 5 mm opening and includes a valve promoting only left-to-right shunting. With the IASD device, right-to-left shunting is prevented by selecting patients with an appropriate left-to-right atrial pressure gradient.
The IASD device is implanted through the femoral vein using a 16 Fr sheath under either local or general anaesthesia. Implantation is guided by transoesophageal or intracardiac echocardiogarphy. After standard transseptal puncture in the mid fossa, the delivery catheter containing the device is advanced to the left atrium over a stiff guidewire. Here, the left side of the IASD is opened and retracted to make contact with the atrial wall. Subsequently, the right side of the device is deployed and the catheter and wire removed. Theoretically, creating an ASD introduces a risk of thromboembolism and the devices require some antithrombotic therapy. Patients implanted with the IASD device have been treated with clopidogrel for six months and aspirin.
So far, the published experience with the devices is limited. For the Corvia IASD device a pilot study (11 patients) and a larger (64 patients, six-month follow-up) phase 1 study have been published5,6. The published experience with the V-Wave device is smaller and limited to a case series and a study in which it was implanted in 10 HFrEF patients7. The primary outcomes in the trials focused on safety. Data on both procedural safety and short-term safety are pivotal in establishing whether this treatment modality would have a future place in HF management. In the largest published material with the Corvia IASD device, no major periprocedural events or fatalities after six months were observed6. One-year data from the pilot trial with the Corvia IASD device were equally encouraging without any patients developing strokes or pulmonary hypertension8. With the V-Wave device no periprocedural events were reported but one death occurred during follow-up7.
Given the limited size of the pilot trials, efficacy focus has been on haemodynamic measures and functional outcomes such as the six-minute walk test (6MWT), exercise capacity, NYHA class, and quality of life (QOL) questionnaires. With both devices, reduction in resting5,7 or exercise6 pulmonary capillary wedge pressure has been reported. Also, device implantation has been associated with significant improvement in NYHA class, 6MWT and measures of QOL5-7.
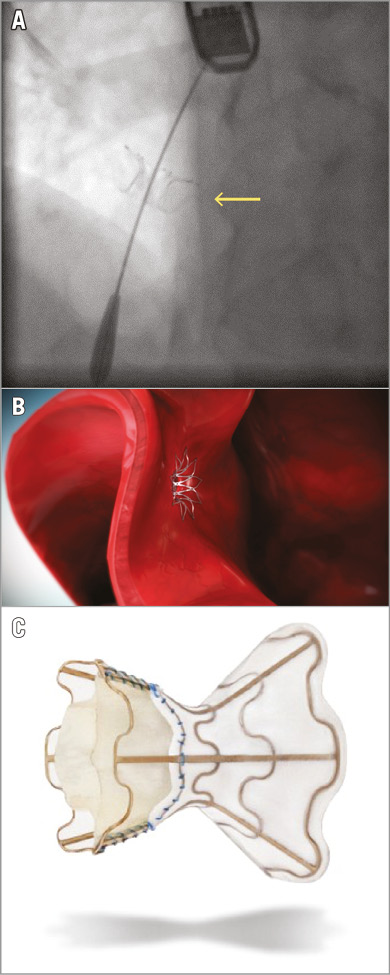
Figure 1. The Corvia and V-Wave devices. A) A Corvia IASD device in situ in the atrial septum (arrow). The tip of the guidewire is in the left atrium. B) The Corvia IASD seen from the left atrial side. C) The V-Wave device.
Haemodynamic measurements as well as exercise-related endpoints are subject to bias in unblinded trials. Experience from earlier trials addressing other catheter-based treatments, such as renal denervation, has taught us the importance of adequate study design when testing medical devices and interventions. The SYMPLICITY HTN-3 trial showed that sham procedures and a double-blinded design changed the perspective on the efficacy of renal denervation and introduced uncertainty about the treatment. To avoid this uncertainty, randomised, double-blinded trials with sham procedures to assess properly the efficacy of these types of treatment are desirable. A US-based trial of the Corvia IASD device using this design has just been initiated and the first patient has been enrolled.
Therapeutic right-to-left shunts
Another population that has been the focus of atrial septostomy is that of patients with severe pulmonary arterial hypertension (PAH). Here the shunt works as a right-to-left shunt, alleviating RV strain, but will invariably induce hypoxaemia which may be especially detrimental in these patients, and may even increase mortality9,10. The current knowledge of iatrogenic ASD to PAH patients is based primarily on retrospective analyses of single-centre experiences with balloon septostomy, in most cases used as a bridge to lung transplantation. To our knowledge, no studies are prospectively enrolling PAH patients for atrial septal device treatment. Whether the newly developed devices might be used for creating predictable right-to-left shunts in severe pulmonary hypertension remains to be seen, but this might be an attractive option in selected cases.
Conclusion
Therapeutic creation of atrial shunts is a novel concept currently being tested in trials of patients with heart failure and elevated left-sided filling pressures. Results so far are encouraging; however, further safety and efficacy data are needed as is definition of the optimal patient characteristics for this intervention.
Conflict of interest statement
F. Gustafsson is an (unpaid) advisor to Corvia Medical. E. Wolsk has no conflicts of interest to declare.

