Abstract
The creation of an interatrial shunt in order to decompress the right or left atrium in patients with right and left ventricular failure, respectively, has been used as an alternative therapy to improve symptoms and clinical outcomes in patients with pulmonary hypertension-right heart failure and left heart failure refractory to optimal medical therapy. If ongoing randomised clinical trials further substantiate these beneficial effects in patients with chronic HF, interatrial shunting will represent an important new approach for treating this population.
Introduction
The creation of an interatrial shunt in order to decompress the right or left atrium in patients with right ventricular (RV) and left ventricular (LV) failure, respectively, has been used as an alternative therapy in patients with pulmonary hypertension (PH)-right heart failure (HF) and left HF1,2,3. This review aims to provide an updated overview and clinical perspective on interatrial shunting for treating different HF conditions, as well as highlighting the potential challenges and future directions of this therapy. A computerised search was performed to identify all relevant studies from PudMed and EMBASE databases. The following terms were used: “interatrial shunt”, “atrial shunting”, “balloon septostomy”, “atrial septostomy”, “atrial decompression”, “interventional therapy pulmonary hypertension”, and “interventional therapy heart failure”. Databases were last accessed in November 2018. In addition to the computerised search, we manually reviewed the bibliography of all included articles to ensure complete inclusion of all possible studies.
INTERATRIAL SHUNTING FOR PULMONARY ARTERIAL HYPERTENSION – RIGHT HEART FAILURE
In Europe, the prevalence and incidence of pulmonary arterial hypertension (PAH) has been estimated at 15-60 cases per million and five to 10 cases per million/year, respectively4.
The response to medical treatment in PAH remains somewhat unpredictable, and lung transplantation, with a high number of contraindications and a very limited access, remains the very last recourse for such patients5. Right ventricular dysfunction in patients with PAH is associated with poor short-term prognosis and remains the main cause of death in this population6.
In an animal PAH model, the presence of an atrial septal defect was associated with improved exercise performance and survival7. Also, PAH patients with congenital heart disease and septal defects have a better life expectancy8. The presence of a patent foramen ovale has also been consistently associated with better clinical outcomes in patients with idiopathic PAH9. Also, the presence of an atrial septal defect in patients with mitral stenosis (Lutembacher syndrome) seems to be associated with fewer symptoms and improved outcomes compared to pure mitral stenosis10. In 1983, Rich and Lam performed the first atrial septostomy (AS) to treat refractory PAH using a blade septostomy catheter11. After an initial experience was associated with a high rate of periprocedural complications12,13, the procedure was modified to balloon dilation atrial septostomy (BDAS) which allows a better control of the final atrial septal defect size and has been associated with a significant reduction in procedural complications14,15.
TECHNIQUE
Following transseptal puncture, a balloon dilation of the interatrial septum using a non-compliant balloon is performed14 (Supplementary Figure 1A). The final balloon size is usually ~10 mm (ranging from 4-20 mm), and the decision about the maximum balloon diameter should be based on the avoidance of a massive shunt leading to refractory hypoxaemia and/or acute pulmonary oedema. Avoiding either a drop >10% in SaO2% compared with baseline and/or an increase in left ventricular end-diastolic pressure exceeding 18 mmHg is recommended. Thus, a progressive balloon dilation technique, with small increases in balloon size, checking haemodynamics and SaO2 values after each balloon dilation, should be used. Chronic anticoagulation has been recommended following the procedure3.
HAEMODYNAMIC RESULTS
Table 1 summarises the main results from the largest series on AS in PAH. The immediate haemodynamic effect of AS includes a small but significant reduction in right atrial pressure (RAP) and SaO2 along with an increase in cardiac index (CI)3,12,14,16,17,18,19,20. Pulmonary artery pressure (PAP) and systemic pressure usually remain stable following AS. The degree of haemodynamic change post-AS is mainly related to baseline RAP values and AS size.
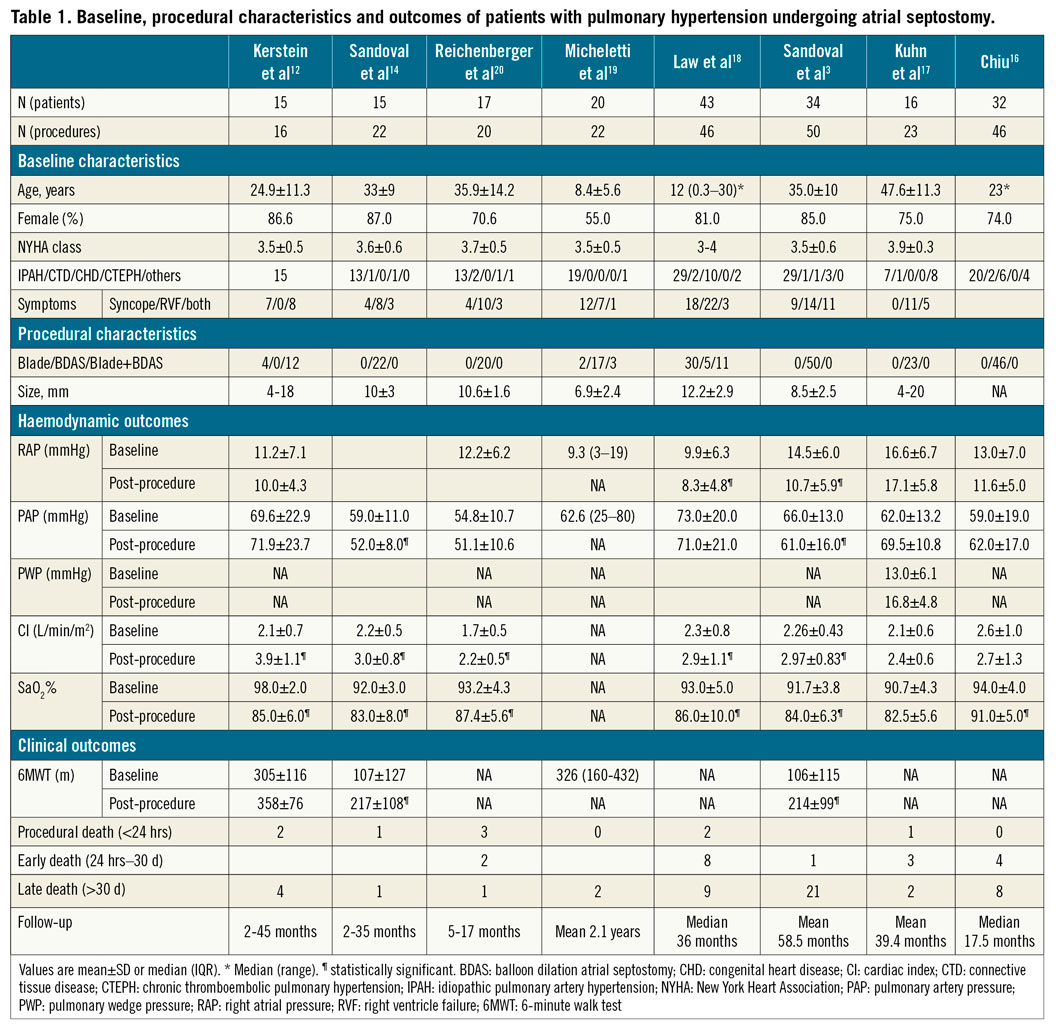
CLINICAL OUTCOMES
Following an initial experience of AS for PAH patients with very high periprocedural mortality rates, there has been a subsequent progressive decrease in procedural mortality (2-7%), mainly related to better patient selection, increased experience of the operators and technical/procedural improvements3,21. Refractory hypoxaemia has been identified as the most common cause of death following AS in PAH patients. Four factors have been identified as potential contraindications for AS: 1) RAP >20 mmHg, 2) SaO2 <90% without supplemental oxygen, 3) left ventricular end-diastolic pressure >18 mmHg, and 4) severe RV failure on cardiorespiratory support21,22,23.
A significant functional class improvement of at least one degree was observed in about 70% of patients and better exercise capacity (mean increase of ~90 metres during 6-minute walk test [6MWT]) have been demonstrated following AS in PAH patients3,12,14,21 (Supplementary Figure 2).
The impact of AS on long-term survival of PAH patients is difficult to establish due to the lack of randomised controlled studies. Some studies comparing AS-PAH recipients with historical series or estimated survival rates have suggested a survival benefit of AS at one- to three-year follow-up3,12,14,16,18 (Supplementary Figure 3). Thus, AS may be considered in patients who are still in World Health Organization functional class (WHO-FC) III or IV despite optimal medical therapy or with severe syncopal symptoms.
SHUNT PATENCY AND PERMANENT DEVICES
The rate of shunt occlusion following BDAS has been close to 20% after a mean follow-up of 15 months3,12,14,17,18,20. Redo AS has been shown to be feasible and safe3,20, and several permanent interatrial shunt devices have been tested in patients with PAH to improve shunt patency and maintain shunt size.
The modified stent, with a diabolo or butterfly shape (Figure 1A), is made by a loop of a pre-defined diameter created with suture or pacing wires placed over the mid portion of the balloon, and a stent mounted and crimped with the loop in the centre of the stent. A series including 12 patients has shown haemodynamic results similar to those obtained with BDAS; no evidence of shunt occlusion was observed after a mean follow-up of two years24.
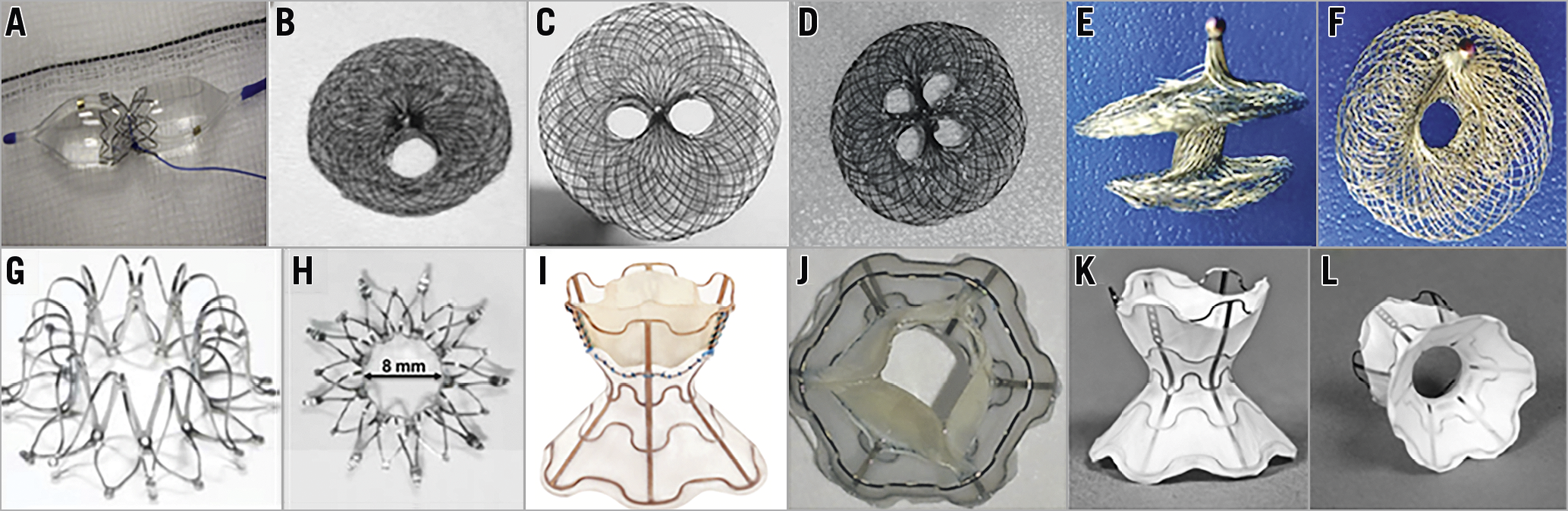
Figure 1. Interatrial shunt devices. A) Diabolo stent. B), C) & D) AMPLATZER Atrial Septal Occluder (ASO) with modified fenestration and different configurations. E) & F) Atrial Flow Regulator (AFR; Occlutech). G) & H) InterAtrial Shunt Device (IASD; Corvia Medical Inc.). I) & J) V-Wave device (V-Wave Inc.). K) & L) Second-generation (valveless) V-Wave device (V-Wave Inc.).
Some studies have shown the feasibility and preliminary efficacy of the AMPLATZER™ Atrial Septal Occluder (St. Jude Medical/Abbott Vascular, Abbott Park, IL, USA) with modified fenestration, including different configurations (Figure 1B-Figure 1D). Contradictory data have been reported regarding midterm to long-term patency25,26,27, and an occlusion rate of up to 40% was reported after a mean follow-up of about one year in a series including 10 patients.
A new device, the Atrial Flow Regulator (AFR; Occlutech, Jena, Germany), consists of a double disc device made of a nitinol wire mesh and a central orifice (Figure 1E, Figure 1F). The fenestration diameter varies from 4 to 10 mm, and there are three waist sizes (2, 5, and 10 mm) to suit the atrial septal thickness. The most important difference between the AFR and the fenestrated AMPLATZER devices is the absence of fabric. A recent study including 12 patients who had AFR implantation and a mean follow-up of about six months showed immediate haemodynamic and clinical improvements similar to BDAS. The permeability of the shunt was demonstrated by contrast echocardiography and oximetry after exercise in all patients28.
ONGOING AND FUTURE STUDIES
Supplementary Table 1 summarises ongoing and future studies.
Interatrial shunting for left heart failure
Despite decades of major advances in medical and device treatment, left HF morbidity and mortality remain high, regardless of aetiology. In patients with chronic HF, increased left atrial (LA) pressure leading to pulmonary congestion is the common mechanism precipitating symptom worsening and acute decompensation29. An interatrial shunt may relieve the volume excess from the left atrium, regulated by the interatrial pressure gradient.
DEVICES
The InterAtrial Shunt Device (IASD®; Corvia Medical Inc., Tewksbury, MA, USA) consists of a nitinol mesh with multiple legs and radiopaque markers, and a central hole for creating the interatrial septal defect (Figure 1G, Figure 1H). When fully expanded, the external and inner diameters are 19 mm and 8 mm, respectively30.
The V-Wave device (V-Wave Inc., Caesarea, Israel) is an hourglass-shaped device made of nitinol with expanded polytetrafluoroethylene encapsulation, and three porcine pericardial leaflets sutured inside to ensure an unidirectional left-to-right shunt (Figure 1I, Figure 1J). The lumen diameter of the V-Wave device is 5 mm1. The newer-generation device is similar but without valve leaflets (valveless device) (Figure 1K, Figure 1L).
The AFR device (previously described and initially tested in patients with PAH) (Figure 1E, Figure 1F) will be tested soon in patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF).
Overall, shunt device size in HF patients has ranged from 5 to 8 mm. Congenital data showed the lack of negative haemodynamic effects among patients with atrial septal defects <10 mm and, in a validated cardiovascular simulation model, Kaye et al31 showed the lack of increase in right atrial and PA pressures with an 8-9 mm shunt. However, further studies are needed to determine the optimal shunt size for patients with left HF.
TECHNIQUE
Following transseptal puncture, a sheath (14 to 16 Fr) is advanced into the LA cavity, and the device deployed using a dedicated delivery system. Of note, balloon predilation is recommended before the implantation of some devices (e.g., AFR device). The left side of the device is initially opened, and the entire system is pulled back ensuring back tenting at the level of the interatrial septum. Then, the right side of the device is deployed, and the device finally released (Supplementary Figure 1B).
Aspirin (75-325 mg daily) indefinitely associated with a P2Y12 inhibitor or anticoagulant therapy (warfarin or a direct-acting oral anticoagulant) for six months has been recommended (empirically) post-procedure.
CLINICAL AND HAEMODYNAMIC RESULTS
The main results of interatrial shunt studies in patients with HFrEF and HFpEF are summarised in Table 2. The first experience using the IASD was in 11 patients with LVEF >45% and NYHA Class III/IV. The device was successfully implanted in all patients and there was a significant decrease in pulmonary capillary wedge pressure (PCWP) with no changes in RAP or PAP and significant improvements in 6MWT distance, quality of life, and NYHA class at 30-day follow-up30.
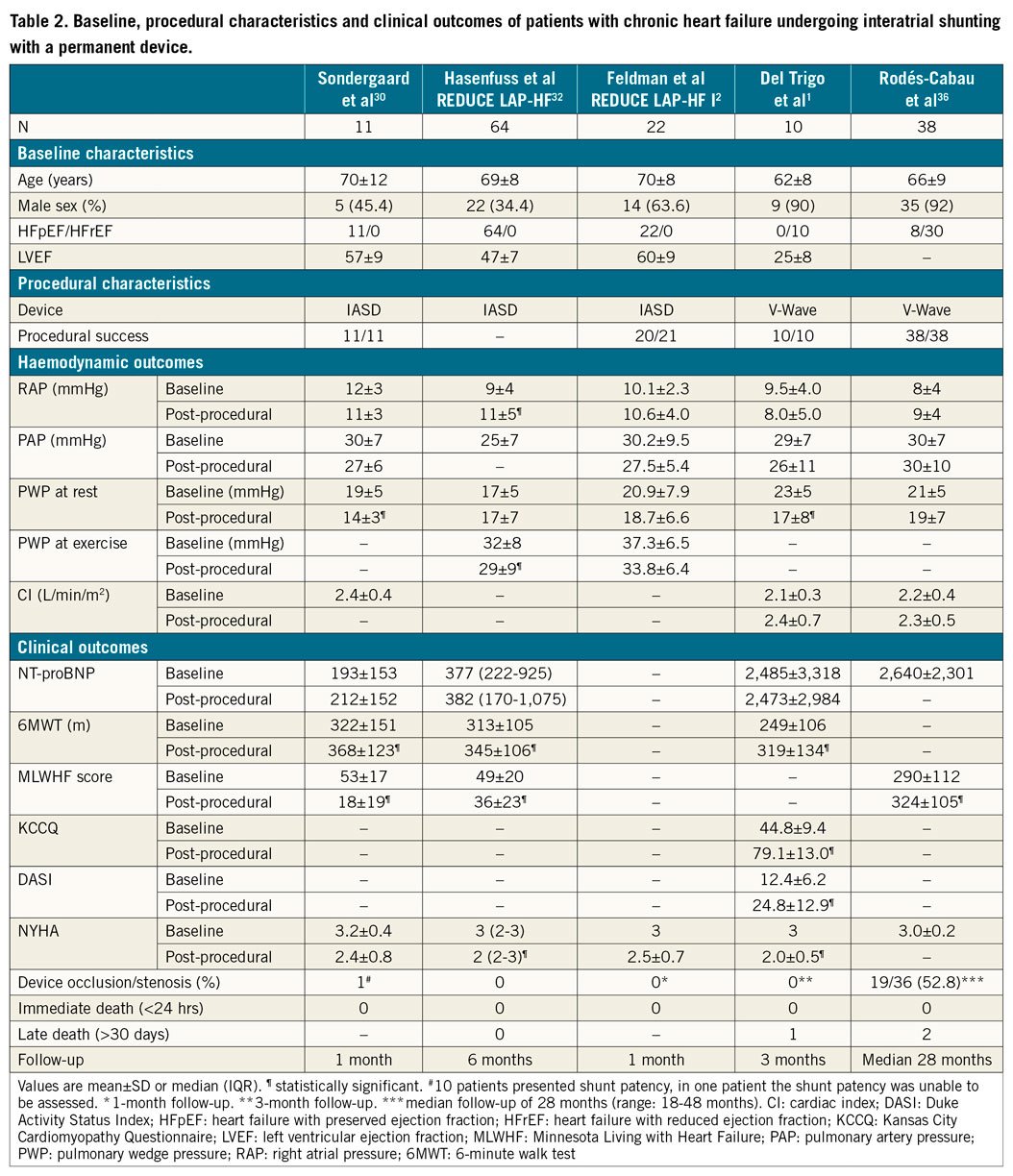
The REDUCE LAP-HF trial32, included 64 patients with symptomatic HFpEF treated with the IASD device with no major periprocedural complications. At six-month follow-up, there were no significant changes in PCWP at rest; a significant decrease in PCWP at peak exercise was observed (p=0.01). Furthermore, improvements in NYHA class, quality of life and exercise capacity were observed at six months32 and maintained at one-year follow-up33.
A randomised controlled trial2 included 44 patients with LVEF >40% and NYHA Class III-IV. At one-month follow-up, patients in the IASD group exhibited a reduction of PCWP values during exercise compared to a lack of changes in the control (no device) group (p=0.01) (Supplementary Figure 4, Table 2). However, these haemodynamic differences between groups did not translate into differences in functional status or exercise capacity at one-month follow-up. Recently, Shah et al34 reported the one-year results, with no evidence of device occlusion over time. Some degree of RV dilation was observed at six months in the group which received the IASD device, with no further RV dilation and no decrease in RV function up to one year. The improvement in quality of life and exercise capacity was similar in both groups, and the IASD group exhibited a tendency towards a better NYHA class improvement (p=0.08) and fewer heart failure hospitalisations (p=0.06). Finally, major adverse cardiac, cerebrovascular or renal events were similar between the groups, with a survival rate of 95% at 12 months (one death in each group)34.
The V-Wave device was the first interatrial shunt device implanted in patients with HFrEF35. Del Trigo et al1 reported the initial experience in 10 patients with HFrEF and functional class III/IV despite optimal medical/device therapy (Table 2). Recently, the results of multicentre initial experience with the V-Wave device36 including 38 patients (HFrEF: 30; HFpEF: 8; NYHA Class III-IV in all of them) were reported. The V-Wave device was successfully implanted in all cases with only one major periprocedural complication (cardiac tamponade resolved with pericardiocentesis). Significant improvements in functional class, exercise capacity and quality of life were observed early after the procedure (within the first three months) and maintained at one-year follow-up. There were no changes in haemodynamic parameters (as determined at rest) at one-year follow-up (Table 2). After a median follow-up of 28 months, 10 patients (26%) had died (eight from cardiovascular causes), one patient received a left ventricular assist device as destination therapy at 15 months, and another underwent heart transplantation at 27 months.
AS has demonstrated clinical benefits and may be considered in patients who remain symptomatic despite optimal medical/device therapy based on current guidelines. Patients with severe RV dysfunction or severe pulmonary hypertension exhibit a higher risk of periprocedural and midterm complications and have been excluded from trials.
SHUNT PATENCY
One of the potential concerns for permanent interatrial shunt devices is shunt patency over time. Kaye et al33 reported shunt patency data at one-year follow-up as evaluated by TTE in 64 patients with HFpEF following the implantation of the IASD device. TTE images were not considered adequate for determining shunt patency in 16 patients (25%), highlighting the potential difficulties in appropriately evaluating the interatrial shunt by TTE. The shunt was patent in all patients with appropriate TTE images (48 patients).
The patency of the V-Wave device was evaluated by TEE at one to three months and at one year after device implantation36. All shunts were fully patent at one to three months, and shunt occlusion was observed in 14% of patients at one-year follow-up. Additionally, some degree of shunt stenosis at the valve level occurred in 36% of patients, leading to an incidence of shunt stenosis or occlusion of 50% at one year. The potential cause of stenosis or occlusion was suggested from a stenotic shunt that was explanted during cardiac transplantation two years after the procedure. The bioprosthetic leaflets were thickened and stenotic with neoendocardial hyperplasia (pannus). These data along with the lack of thrombus suggested intra-shunt valve deterioration as the main mechanism of shunt stenosis-occlusion. This is the reason why a newer generation of the V-Wave device has been developed.
Comparative analysis of haemodynamic and clinical outcomes between patients with and without shunt stenosis or occlusion showed that patients with fully patent shunts exhibited significant improvements in haemodynamic parameters compared to a lack of changes in the shunt stenosis-occlusion group. Also, those patients with patent shunts had improved late clinical outcomes, with lower rates of death/left ventricular assistance/transplantation or heart failure rehospitalisation at three-year follow-up (Supplementary Figure 5),36.
FUTURE STUDIES
Supplementary Table 1 summarises the ongoing and future studies.
Clinical implications and future directions
Current evidence for interatrial shunting is based on observational studies and small randomised trials showing the feasibility, safety and preliminary efficacy in patients with PAH and left HF. These data seem to be insufficient to modify current clinical practice but support the use of interatrial shunting as a palliative therapy in selected patients with PAH and left HF who remain symptomatic despite optimal treatment based on current guidelines. Several ongoing randomised trials will provide definite evidence about the exact role of this therapy for the treatment of HF patients. If further substantiated and associated with improved clinical outcomes, device-mediated left-to-right atrial shunting would offer an important new approach to treatment of this population.
Conclusions
In PAH patients, AS can be considered as a palliative therapy in non-responders to available therapies. Careful patient selection and limiting the size of the atrial septal defect appear to be key in order to avoid major periprocedural complications and death. Also, new permanent devices are currently being evaluated in order to ensure shunt patency over time. In left HF patients, interatrial shunting with different permanent devices has been shown to be a feasible and safe therapy in patients with chronic left HF who remain symptomatic despite optimal medical/device therapy. Also, preliminary efficacy data, with significant improvements in functional status, exercise capacity and quality of life, have provided the rationale for designing large randomised trials (currently ongoing) in order to determine further the efficacy of this new therapy for reducing major cardiovascular events in patients with chronic HF.
Acknowledgements
J. Rodés-Cabau holds the Research Chair “Famille Jacques Larivière” for the Development of Structural Heart Disease Interventions.
Conflict of interest statement
J. Lindenfeld, A. Bayés-Genis, and J. Rodés-Cabau are consultants for V-Wave Inc. The other authors have no conflicts of interest to declare.

