Abstract
Aims: The aim of this study was to evaluate the effect of the creation of a left-to-right interatrial shunt on pulmonary haemodynamics in rats with heart failure with preserved ejection fraction (HFPEF).
Methods and results: An interatrial communication (IAC) was created in 11 healthy rats (Lewis rats) and 11 rats which developed HFPEF (36-week-old spontaneously hypertensive rats [SHR]). Effects of the interatrial shunt were compared to 11 sham-operated Lewis and 11 sham-operated SHR. At 45 days post shunt, strain effect was observed in diastolic function (E/A ratio, p<0.001; isovolumetric relaxation time, p<0.001), left atrial volume (p=0.005) and pulmonary wall shear rate (WSR) (p=0.02) measured by Doppler echo. At sacrifice of the animals (60 days), a strain effect was also noted in elastin density (p=0.003) and eNOS protein expression (p=0.001). Interatrial shunt creation resulted in (i) an increase in pulmonary WSR (p=0.04) and a decrease in left atrial volume (p<0.001), (ii) an increase in elastin density (p<0.005), and (iii) an increase in eNOS protein expression (p=0.03).
Conclusions: Creation of a left-to-right atrial shunt in rats with HFPEF was effective in improving pulmonary haemodynamics. In addition, this study provides preliminary evidence of the potential risk of right volume overload and pulmonary hypertension due to atrial shunting.
Introduction
Heart failure is an increasingly common disease related to population ageing1. In clinical nosology, cardiac failure is identified either with a decrease in left ventricular ejection fraction (LVEF) or with a preserved ejection fraction (heart failure preserved ejection fraction [HFPEF]), always associated with an increase in telediastolic pressure2 and left ventricular hypertrophy. The incidence of HFPEF is constantly increasing and represents up to 50% of patients with heart failure3. However, pharmacological treatment does not improve survival4, in contrast to what is observed in heart failure with decreased ejection fraction; alternative therapy is eagerly awaited.
Ben Driss et al studied the haemodynamic phenomena associated with HFPEF caused by a small left ventricular infarct in rats in an experimental model5. In this model, while there was no change in cardiac output or peripheral arterial circulation, Doppler ultrasound demonstrated an impact on the pulmonary circulation. Increase in pulmonary pressure is responsible for passive dilation of the pulmonary arteries (PA) and veins, because of the high capacitance of these low-pressure pulmonary arterial and venous circulations6. As a result, endothelial wall shear rate (WSR) is reduced in the pulmonary circulation. Decrease in WSR alters endothelial function in the pulmonary circulation, including decreasing NO synthase expression and a tendency to increase endothelin7 and IL-68 expression and secretion. If the pressure stimulus persists, functional and structural remodelling occurs in the pulmonary arterial circulation, leading to pulmonary hypertension and right heart failure9.
Due to this pulmonary impact related to the increase in diastolic pressure above the left ventricle (LV), the creation of a shunt between the left and right atria in the clinical setting was recently proposed10. In the context of heart failure, this shunt decreases left atrial and pulmonary arterial pressures, at rest as well as during exercise, which improves symptoms during exercise (dyspnoea on exertion). The effect of an interatrial shunt on pulmonary haemodynamics has not been studied in experimental HFPEF to date.
The aim of this study was to develop a new model of interatrial shunt in rats, and to evaluate the effect of this left-to-right shunt on pulmonary haemodynamics in HFPEF. For this purpose, a left-to-right shunt was created (interatrial communication [IAC]) in normotensive healthy inbred Lewis rats and spontaneous hypertensive rats (SHR). SHR develop compensated heart failure (HFPEF) in the first year of life, before dying at two years with decompensated cardiac heart failure11. The impacts of rat strain and of the interatrial shunt were evaluated using Doppler echocardiography and biological analyses at sacrifice.
Materials and methods
EXPERIMENTAL DESIGN
Four groups of animals were studied according to an experimental protocol of Latin square design - two groups of 11 inbred Lewis rats 11 weeks old weighing 400±25 gr (group 1 was sham-operated whereas IAC was created in group 2), and two groups of 11 inbred SHR 36 weeks old, of identical weight (all from Envigo, Gannat, France) (group 3 was sham-operated whereas IAC was created in group 4).
Thirty-six-week-old SHR are hypertensive rats which develop HFPEF without systolic dysfunction12. In addition, the SHR strain was derived from the Wistar rat strain13.
EXPERIMENTAL RAT MODEL OF IAC
The rats were anaesthetised with isofluorane 4%, then intubated and ventilated. Anaesthesia was maintained by isofluorane 2%. After right thoracotomy and pericardiectomy, a purse-string suture was performed on the right atrium using an 8/0 polypropylene wire. A micro-balloon catheter (Mini Trek®, 2 mm in diameter, 8 mm in length; Abbott Vascular, Santa Clara, CA, USA) was introduced through this purse string to perforate the interatrial septum. Then, the purse string was tightened simultaneously to catheter removal. Haemostasis was completed using SURGICEL® (Cordis, Cardinal Health, Milpitas, CA, USA) and simple stitches of 8/0 polypropylene when necessary. At the end of the intervention, the rats were subjected to volume expansion by receiving 10 mL of NaCl 0.9% subcutaneously together with an injection of analgesic (buprenorphine 0.05 mg/kg subcutaneously). The sham-operated rats underwent a similar procedure without creation of an IAC.
The project was evaluated by the Animal Experiments Ethics Committee N°121 (French Ministry of Higher Education, Research and Innovation) and received a favourable decision. The project authorisation reference number was APAFIS#12184- 20171115151515174156 v6.
DOPPLER ECHOCARDIOGRAPHIC STUDY 45 DAYS AFTER OPERATION
Forty-five days after surgery, the rats were anaesthetised with 2% isofluorane in order to perform Doppler echocardiography (Vevo® 2100, probe MS-200; VisualSonics, Toronto, ON, Canada). In the parasternal and apical sections we measured the following: (i) diameter and maximal systolic velocity of the PA, as measured 10 mm downstream of the pulmonary valve, allowing calculation of WSR in the PA (in s-1) (Figure 1), the formula used to calculate endothelial WSR in the PA being τ = (4x max. systolic velocity)/(PA systolic diameter)14; (ii) diastolic function evaluated by measurement of the transmitral Doppler E/A ratio and by measurement of isovolumetric relaxation time (IVRT in ms)15; (iii) ejection fraction obtained by calculating the Teichholz index; (iv) systemic cardiac output (in mL/mn) calculated from cardiac frequency (CF), velocity time integral (VTI) and diameter of outflow tract of the LV (d) using the formula Πd2/4xITVxCF; and (v) anteroposterior diameter of the left atrium (D), as measured next to the aortic valve, permitting calculation of the left atrial volume (in mm3) using the formula Π/6 × D3.
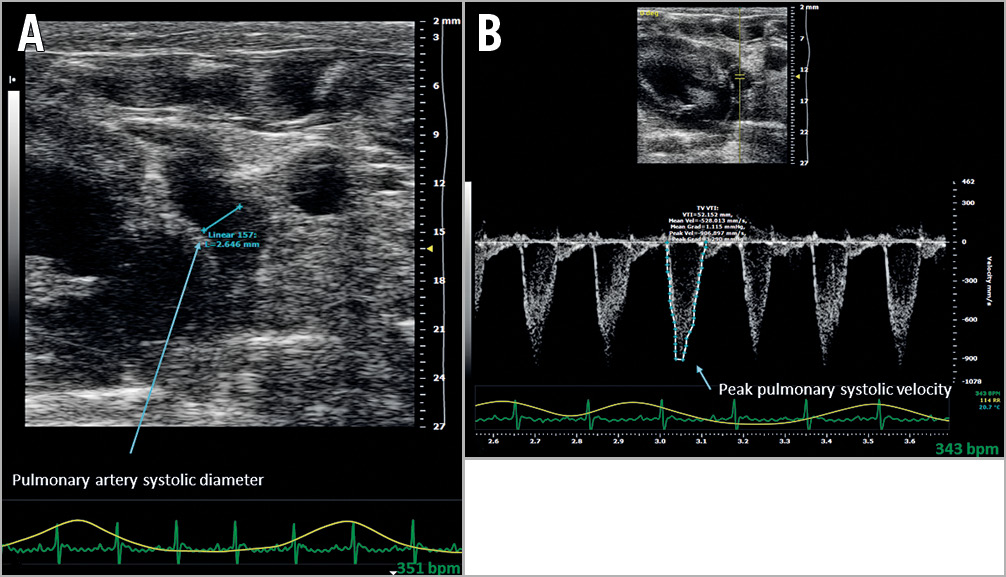
Figure 1. Doppler ultrasound wall shear rate measurement in the pulmonary artery. (τ =[4x max. systolic velocity]/[pulmonary artery systolic diameter]) at 45 days. A) Measurement of pulmonary artery systolic diameter, as measured 10 mm downstream of the pulmonary valve. B) Measurement of maximum systolic velocity in the pulmonary artery, as measured 10 mm downstream of the pulmonary valve.
MORPHOLOGICAL STUDY
Sixty days after the operation, the animals were weighed and then sacrificed under anaesthesia (pentobarbital 0.2 mg/kg intraperitoneal). Hearts and lungs were harvested and weighed separately. Patency of IAC was verified visually after opening the left and right atria. Rats presenting a non-patent IAC were excluded from the study. PA, LV, right ventricle and right and left atria were dissected out and weighed. The PA was removed and fixed in 10% formaldehyde, then embedded in paraffin, and sections of 5 μm thickness were used for histological analysis. After staining with orcein, a morphometric study of the PA was performed, including quantification of media thickness and elastin. Elastin density (%) was obtained by calculating the ratio between elastin density and media thickness.
ATRIAL NATRIURETIC PEPTIDE (ANP)
A blood sample was collected on ethylenediamine tetraacetic acid (EDTA) during sacrifice to measure the plasma ANP concentration, using an ELISA kit (RayBiotech, Peachtree Corners, GA, USA) according to the manufacturer’s instructions.
WESTERN BLOT OF eNOS
eNOS is a marker of endothelial WSR16. At sacrifice, a lung fragment was directly frozen and then pulverised using liquid nitrogen with mechanical stirring. On this sample, eNOS protein expression was quantified in a western blot analysis (Supplementary Appendix 1).
STATISTICAL ANALYSIS
Continuous variables were expressed as means±standard deviation. Normal distribution was verified using the Kolmogorov-Smirnov test, and homogeneity of variance was tested by Levene’s test. Two-factor analysis of variance (interatrial shunt/strain factor, two-way ANOVA) was performed for testing the effect of strain and the effect of the shunt. Distribution of PA systolic diameter followed a normal distribution; therefore, inter-group comparison for this parameter was made using the Student’s t-test. A p-value of <0.05 was considered significant. All calculations were made using SPSS software, Version 20.0.0.0 (IBM Corp., Armonk, NY, USA). Figures were created using GraphPad Prism v7.0 (GraphPad Software, La Jolla, CA, USA).
Results
RAT CHARACTERISTICS (Figure 2, Supplementary Table 1)
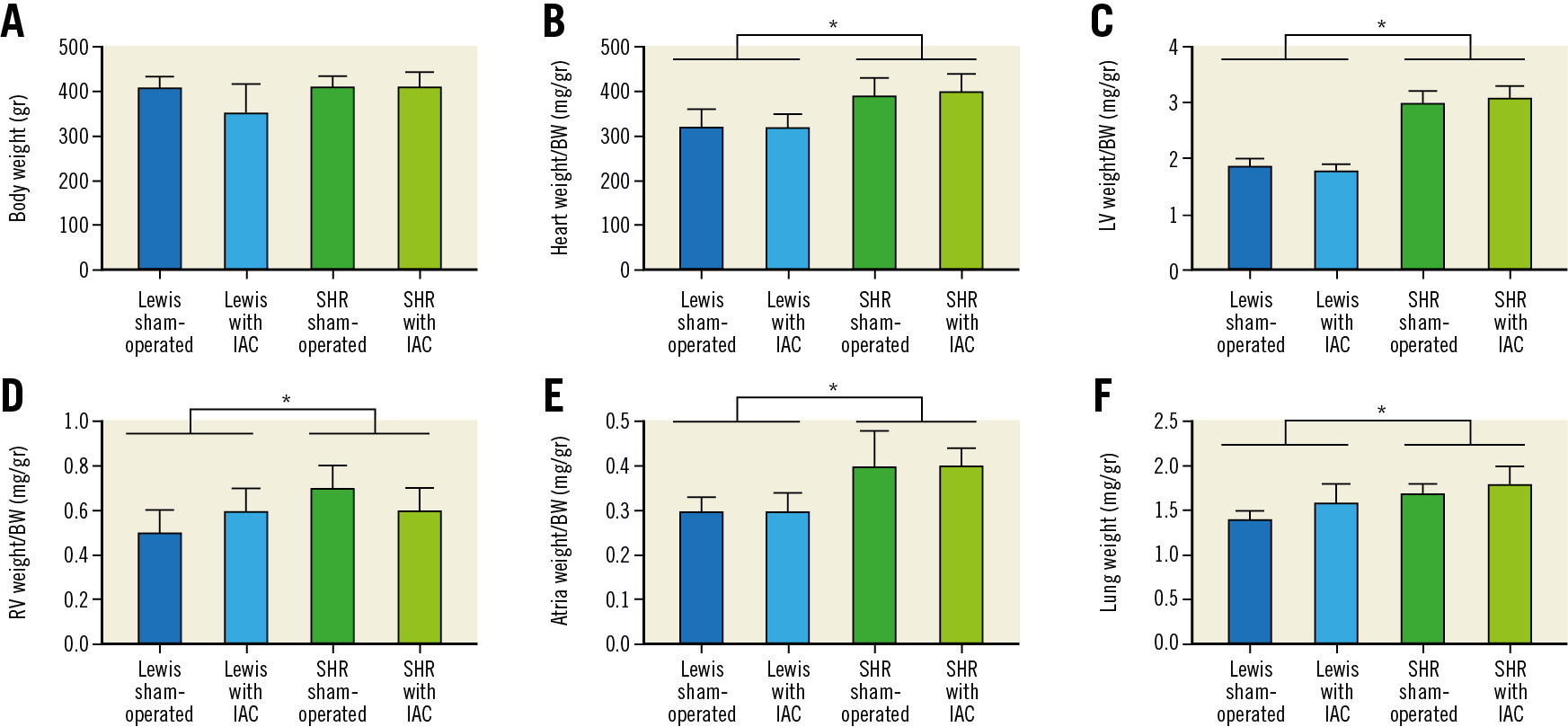
Figure 2. Weights of body, heart and lungs. A) Body weight, gr. strain effect: F=2.4, p=NS. IAC effect: F=3.6, p=NS. B) Heart weight/body weight, mg/gr. strain effect: F=52.3, *p<0.001. IAC effect: F=0.2, p=NS. C) Left ventricle weight/body weight, mg/gr. strain effect: F=6.41, *p<0.001. IAC effect: F=0.2, p=NS. D) Right ventricle weight/body weight, mg/gr. strain effect: F=23.2, *p<0.001. IAC effect: F=0.1, p=NS. E) Atria weight/body weight, mg/gr. strain effect: F=14.0, *p<0.001. IAC effect: F=0.9, p=NS. F) Lung weight, gr. strain effect: F=27.5, *p<0.001. IAC effect: F=3.5, p=NS. BW: body weight; IAC: interatrial communication; LV: left ventricle; NS: non-significant; RV: right ventricle; SHR: spontaneous hypertensive rats
Forty-six rats were operated; two Lewis rats had a non-permeable IAC and were excluded from the investigation. In total, 11 rats were included in each group. A strain effect was noted for the weights normalised to body weight for lungs (SHR >Lewis, F=27.5, p<0.001), whole heart (SHR >Lewis, F=52.3, p<0.001), LV (SHR >Lewis, F=641.1, p<0.001), right ventricle (SHR >Lewis, F=23.2, p<0.001) and the right and left atria (SHR >Lewis, F=14.0, p=0.001). Creation of IAC did not change body, heart or lung weights (IAC vs sham). The weight of Lewis and SHR was similar 60 days after intervention (430±52 versus 412±28 gr, p=0.145).
DOPPLER ECHOCARDIOGRAPHY (Figure 3, Supplementary Table 2)
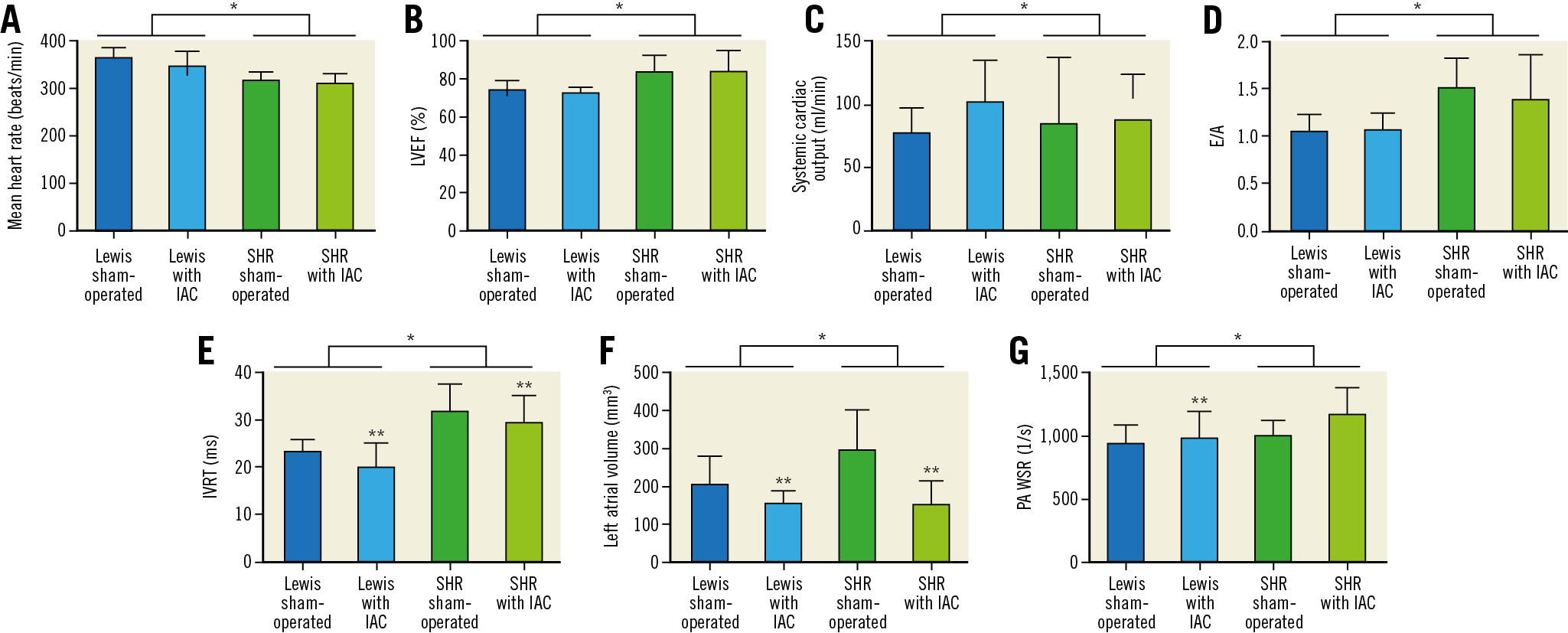
Figure 3. Doppler echocardiography results on systolic function, diastolic function and pulmonary artery wall shear rate (two-way ANOVA). A) Mean heart rate (beats/min). Strain effect: F=33.5, *p=<0.001. IAC effect: F=2.7, p=NS. B) LVEF (%). Strain effect: F=23.7, *p<0.001. IAC effect: F=0.1, p=NS. C) Systemic cardiac output (ml/min). Strain effect: F=0.8, p=NS. IAC effect: F=0.5, p=NS. D) E/A. Strain effect: F=17.3, *p<0.001. IAC effect: F=0.2, p=NS. E) IVRT (ms). Strain effect: F=36.4, *p<0.001. IAC effect: F=3.6, p=NS. F) Left atrial volume (mm3). Strain effect: F=4.5, *p=0.04. IAC effect: F=18.8, **p<0.001. G) PA WSR (1/s). Strain effect: F=5.8, *p=0.02. IAC effect: F=4.3, **p=0.04. IAC: interatrial communication; IVRT: isovolumetric relaxation time; LVEF: left ventricular ejection fraction; NS: non-significant; PA: pulmonary artery; SHR: spontaneous hypertensive rats; WSR: wall shear rate
A strain effect was observed in heart rate (SHR
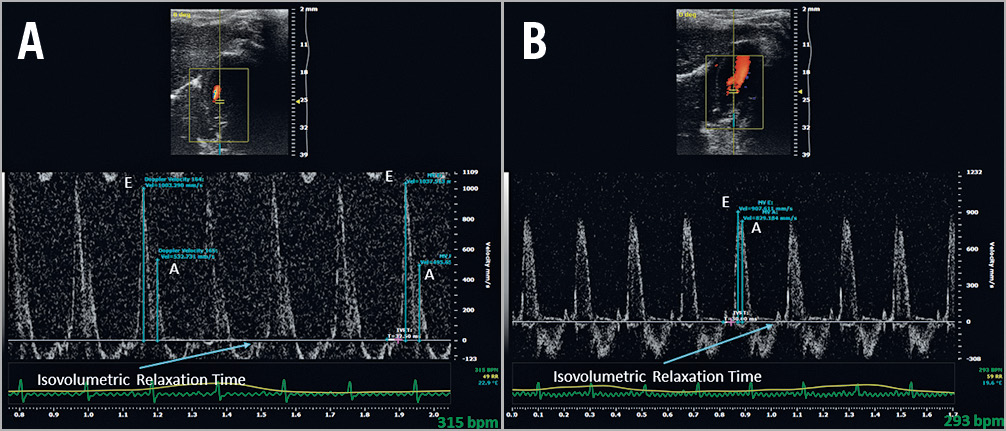
Figure 4. Doppler ultrasound measurement of diastolic function at 45 days (E/A ratio and isovolumetric relaxation time). A) Sham-operated SHR. B) SHR with interatrial communication.
A statistical interaction occurred between rat strain and IAC showing that the left atrial volume decreased more in SHR than in Lewis rats (F=5.1, p=0.03). PA systolic diameter increased in the Lewis strain (2.9±0.2 versus 3.1±0.3, p=0.04) but decreased in SHR (2.9±0.3 versus 2.6±0.1, p=0.001) after IAC creation.
PLASMA ANP CONCENTRATION
ANP concentration was 7.1±5.5 µg/mL in sham-operated Lewis rats, 11.3±10.1 µg/mL in Lewis rats with IAC, 5.6±4.9 µg/mL in sham-operated SHR, and 8.6±3.3 µg/mL in SHR with IAC. There was no significant effect of strain (p=0.29) or shunt (p=0.08) on ANP concentration.
MEASUREMENT OF PA MEDIA THICKNESS AND ELASTIN DENSITY (ELASTIN/MEDIA THICKNESS RATIO) BY QUANTITATIVE HISTOMORPHOMETRY
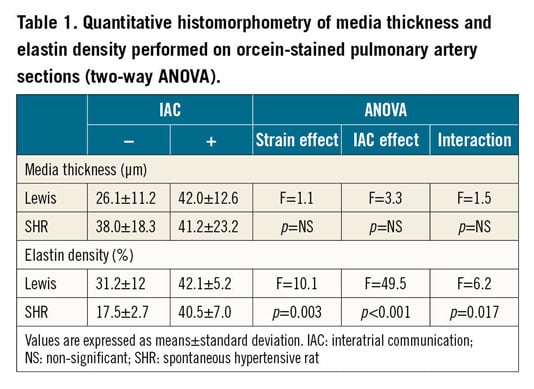
A strain effect was noted in elastin density (SHR >Lewis, F=10.1, p=0.003). Moreover, elastin density increased after creation of the IAC (F=49.5, p<0.001). A statistical interaction occurred between strain and IAC for elastin density (F=6.2, p=0.017), showing that PA elastin density increased more in SHR than in Lewis rats. Examples of orcein staining and results of quantitative histomorphometry are presented in Figure 5 and Table 1.
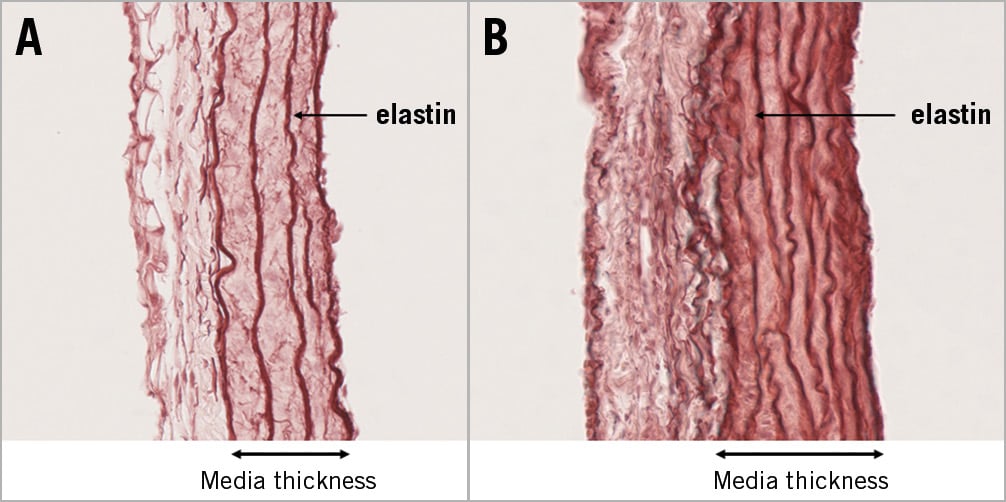
Figure 5. Section of pulmonary artery after staining with orcein (elastin). Media thickness and elastin density were increased in SHR with interatrial communication. A) Sham-operated SHR. B) SHR with interatrial communication.
EXPRESSION OF eNOS PROTEIN BY WESTERN BLOT
eNOS/β-actin ratio was 0.38±0.23 in sham-operated Lewis rats, 0.56±0.36 in Lewis rats with IAC (+47% increase), 0.67±0.17 in sham-operated SHR and 0.87±0.28 (+30% increase) in SHR with IAC. A strain effect (F=12.7, p=0.001) and a shunt effect (F=4.9, p=0.03) were observed, without significant interaction. Results of western blot are illustrated in Figure 6 and Supplementary Figure 1.
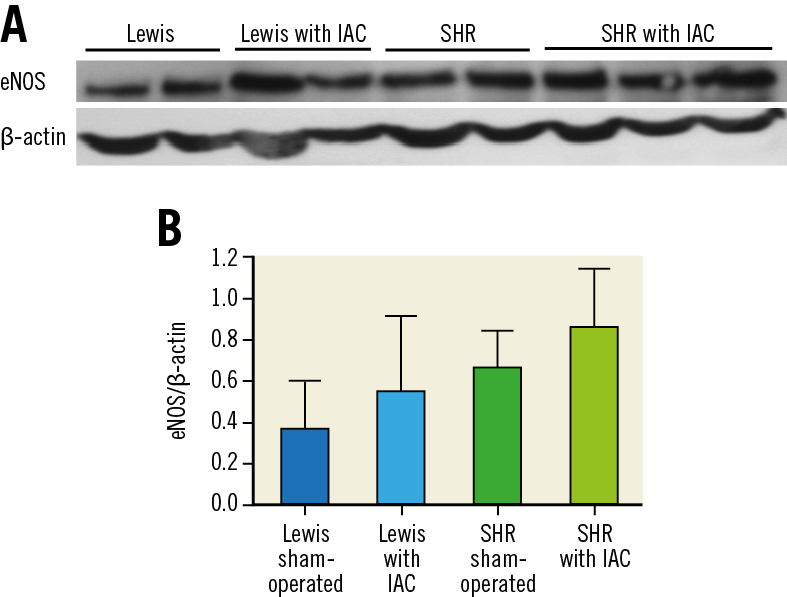
Figure 6. Measurement of eNOS protein expression by western blot in lung fragments. A) Gel representing eNOS protein expression (anti-eNOS and anti-β-actin antibodies). B) Quantification of eNOS normalised to β-actin (ImageJ® software). Interatrial creation increased eNOS/β-actin ratio. A strain effect (F=12.7, p=0.001) and a shunt effect (F=4.9, p=0.03) were observed, without significant interaction.
Discussion
This study showed a strain effect (hypertensive versus normotensive) on heart and lung weights, diastolic function, left atrial volume and pulmonary WSR measured by Doppler ultrasound, elastin density evaluated by histomorphometry and eNOS protein expression. Creation of an IAC in both SHR and Lewis rats decreased left atrial volume and increased pulmonary WSR measured by Doppler ultrasound, providing evidence for the functionality of the shunt. It also tended to improve diastolic function (IVRT), increased media thickness and elastin density of the PA and expression of eNOS protein in the lungs. Wall shear stress is proportional to both viscosity (considered as constant in this case) and velocity and is inversely proportional to the PA dimensions (radius).
In this experiment, changes in the PA diameter associated with interatrial shunting were opposite (interaction, p<0.001) in SHR and Lewis rats. PA diameter increased with shunting in Lewis rats (p=0.05) potentially due to active dilation, whereas it decreased in SHR (p=0.001) potentially due to the pressure decrease. These results suggest that creation of a left-to-right interatrial shunt may be effective in reducing pulmonary capillary pressure in SHR, which may transfer into improvement in HFPEF symptoms, but created a right volume overload in healthy conditions. It is well known that spontaneous atrial septal defects in humans are often benign, but could evolve with time towards right heart failure and pulmonary hypertension17.
There was also a trend towards decreased IVRT (without change in heart rate), suggesting improved diastolic function with IAC in SHR, although this result was not significant. One would have expected a lower ANP concentration in SHR with IAC in keeping with lower left atrial volume, as atrial dilation is the stimulus for ANP secretion18. However, no such difference was found. One hypothesis is that the creation of a left-to-right atrial shunt would increase right atrial volume and pressure (not measured), which would explain the absence of any decrease in ANP concentration.
Several models of left-to-right atrial shunt have been developed in large animals19 but, to our knowledge, no such model has been described in small animals to study HFPEF. Our initial technique consisted of creating a shunt by interposing a syngeneic aortic graft between left and right atrial appendages. This difficult and time-consuming procedure was modified for this study by using a micro-balloon catheter. The systematic study of IAC patency during animal sacrifice confirmed the effectiveness of the procedure (two failures out of 24 IACs created).
Distally ligating the anterior interventricular artery in Lewis rats is a good model for HFPEF20. However, performing this procedure followed by creation of an interatrial shunt resulted in a high death rate (ethical issue). For this reason, we preferred to use SHR with diastolic dysfunction without decreased systolic function (compensated heart failure, HFPEF)21. Thirty-six-week-old SHR develop diastolic dysfunction before the appearance of hypertrophy/hypertension and decrease of LVEF, in contrast to what is observed later in life12. Beyond this age, LV systolic dysfunction appears and rats are likely to die of congestive heart failure11. As normotensive controls, we chose to use inbred Lewis rats, since the usual control rats, Wistar Kyoto, are not an inbred strain (outbred) and body weights are quite different.
The safety and feasibility of creating a left-to-right artificial shunt by performing a percutaneous IAC have been studied in humans22. In a multicentre non-controlled phase 1 study23, 68 patients over 40 years of age with heart failure and LVEF >40% were included. The interventional procedure did not lead to any major cardiovascular or cerebral complications. The percutaneous device was successfully implanted in 64 patients (97%). The first results were encouraging since most patients presented an improvement in NYHA class, dyspnoea, quality of life, exercise capacity, cardiac output and pulmonary arterial oxygen saturation at six months. However, these encouraging clinical benefits were limited24. Indeed, NYHA functional class changed from III to II, and the average distance for the six-minute walk test increased from 313 to 346 metres. A multicentre sham-controlled phase two randomised study25 showed that shunting was associated with a decrease in pulmonary capillary pressure during exercise, providing physiopathological arguments and demonstrating the effectiveness of the procedure. Nevertheless, one month after the procedure, NYHA functional class and exercise duration were similar in the IAC group and the control group. This suggests a potential placebo effect in the phase I study. In addition, the creation of a left-to-right atrial shunt may lead to late right ventricular dysfunction26. Finally, the creation of an artificial left-to-right shunt may improve symptoms of HFPEF, but it is not curative. Thus, it is also essential to find pharmacological and interventional solutions to improve diastolic dysfunction27.
Limitations
This experimental design had some limitations. First, typically age-matched normotensive Wistar Kyoto rats are the controls for SHR28. However, rats were matched on weight rather than age for reproducibility technical reasons. Furthermore, Lewis rats are a pure strain while Wistar Kyoto rats are not. Second, when the IAC was created, the atrial septum was perforated “blindly”. Indeed, the catheter path could be anticipated but the atrial septum was perforated through the right atrium, and it was impossible to verify the reality of the IAC. IAC patency was thus checked during dissection of the heart at sacrifice (day 60), and two Lewis rats with non-patent IAC were excluded. Third, Doppler echocardiography is operator-dependent and requires learning time, and only one Doppler echo measurement was taken for each parameter. Fourth, in SHR, although E/A ratio was greater and IVRT was longer, the mean heart rate was lower as compared to Lewis rats, making the comparison between the two strains difficult. Fifth, even though quantitative histomorphometry provided the expected results (lower elastin density), a limitation of this technique is represented by random sampling. Finally, it was demonstrated that exercise training improved delayed deterioration of cardiac function and was an effective treatment in HFPEF rats29 and humans30. Therefore, it will be important to test the effect of atrial shunting on exercise tolerance in our experimental model. This could be done by using a forced swimming test in rats31. This study was limited to haemodynamic, biochemical and histomorphometric studies of the animals, and therefore improvement in symptoms, exercise tolerance or mortality was not evaluated. This should be the next step, now that we have been able to validate this new animal model.
Conclusions
Creating an IAC with this new model effectively produces a left-to-right atrial shunt in SHR. This technique appears to be effective in improving pulmonary haemodynamics in experimental HFPEF. In addition, our experimental study provides preliminary evidence of the potential risk of right volume overload and pulmonary hypertension due to atrial shunting in healthy rats. Finally, this new preclinical model should be carefully studied.
|
Impact on daily practice Creating a left-to-right interatrial shunt in heart failure with preserved ejection fraction seems to be effective in improving pulmonary haemodynamics. This study could justify and corroborate the further development of a left-to-right interatrial shunt in humans with heart failure with preserved ejection fraction, even though the clinical effects of this shunt remain uncertain. |
Funding
This work was supported by the French Society of Thoracic and Cardiovascular Surgery and by the ADETEC association. The design of the study, collection and analysis of data, and writing and publication of the manuscript were performed independently by the authors, without influence from the sponsors.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.