A 46-year-old African-American female with a history of diabetes, morbid obesity and World Health Organization group IV pulmonary hypertension (PH) secondary to chronic thromboembolic disease was referred to us for atrial septostomy. She was not a candidate for surgical thromboendarterectomy because of the distal location of the thromboembolic disease, nor was she a candidate for lung transplantation due to morbid obesity. She remained symptomatic despite maximal medical therapy including continuous intravenous treprostinil, sildenafil, and bosentan. Due to persistent symptoms of dyspnoea New York Heart Association (NYHA) Class IV with recurrent syncopal episodes on exertion and further deterioration of right ventricular function, we offered her atrial septostomy as palliative treatment.
Graded balloon atrial septostomy is an accepted palliative therapeutic option for patients with severe PH who have significant right ventricular failure, symptoms refractory to maximal medical therapy, and who are not candidates for lung transplantation1. However, spontaneous closure has been reported in 30-54% of published cases, often requiring repeat atrial septostomy2. Techniques aimed at reducing the rate of spontaneous closure are being investigated with the use of fenestrated Amplatzer atrial septal occluder devices or stents. Spontaneous closure of such devices has been observed3, and their long-term safety is unknown.
The patient underwent successful balloon atrial septostomy in April 2011. Three inflations were performed using 4 mm, 6 mm, and 8 mm balloons. Her mean right atrial pressure was reduced from 15 to 10 mmHg, and her cardiac index (CI) at rest increased by 15% from 2.03 to 2.37 L/min/m2. She had excellent clinical response with significant improvement of symptoms (NYHA II). Her six-minute walk distance (6MWD) markedly improved from 120 m before septostomy to 278 m at one month post procedure, and her haemodynamic response to exercise normalised. However, she was admitted at five months with dyspnoea Class III-IV and recurrent presyncope on exertion. She was found to have spontaneous closure of the septostomy and was referred to us for repeat atrial septostomy.
Because her initial septostomy had remained patent for less than five months and she had experienced significant benefit during patency, we contemplated options that might improve patency, including fenestrated atrial septal defect closure devices, stent, or cryotherapy. We considered repeat balloon septostomy with cryotherapy to be the best option because it did not involve implantation of a device.
The theoretical mechanism of action behind cryoplasty therapy is apoptosis. In vitro studies demonstrated that cryotherapy can modulate the neointimal response that leads to restenosis through induction of smooth muscle cell apoptosis. The apoptotic cells suffer controlled regulated death and are phagocytosed by macrophages without causing inflammatory response4. The PolarCath® peripheral balloon dilatation system (Boston Scientific Corp., Natick, MA, USA) has shown favourable clinical outcomes in patients with peripheral arterial disease and during angioplasty for pulmonary vein stenosis. Whether cryotherapy results in local apoptosis at the edges of the atrial septostomy reducing spontaneous closure rates has not been studied.
The off-label use of cryotherapy during atrial septostomy and other alternatives including palliative/hospice service were discussed with the patient. She verbalised understanding and chose repeat septostomy with cryotherapy.
Atrial septostomy was performed in October 2011 using 4 mm, 6 mm, 8 mm, and 10 mm balloons (Figure 1A). We noticed a 25% increase in cardiac output at rest and during arm exercise, and a 6% decrease in systemic oxygen saturation. Intracardiac echocardiography revealed an 8 mm diameter atrial septostomy. We then applied cryotherapy at –10°C with a 7 mm×4 cm PolarCath (Figure 1B). No procedure-related complications occurred.
Her symptoms improved. The 6MWD (which had decreased after spontaneous septostomy closure) improved from 213 m to 259 m at six months and remained stable with 260 m walked during a six-minute walk test (6MWT) at one year. The transoesophageal echocardiogram at one year revealed patent septostomy (Figure 1C), and structural changes in the intra-atrial septum (Figure 1D). There was a honeycomb appearance with two layers of intra-atrial septum encasing an echogenic space without evidence of flow across, probably representing vacuolisation changes related to prior septostomy or cryotherapy. These changes were not noted on prior echocardiograms. Overall, she has survived more than one year after her repeat atrial septostomy without further interventions.
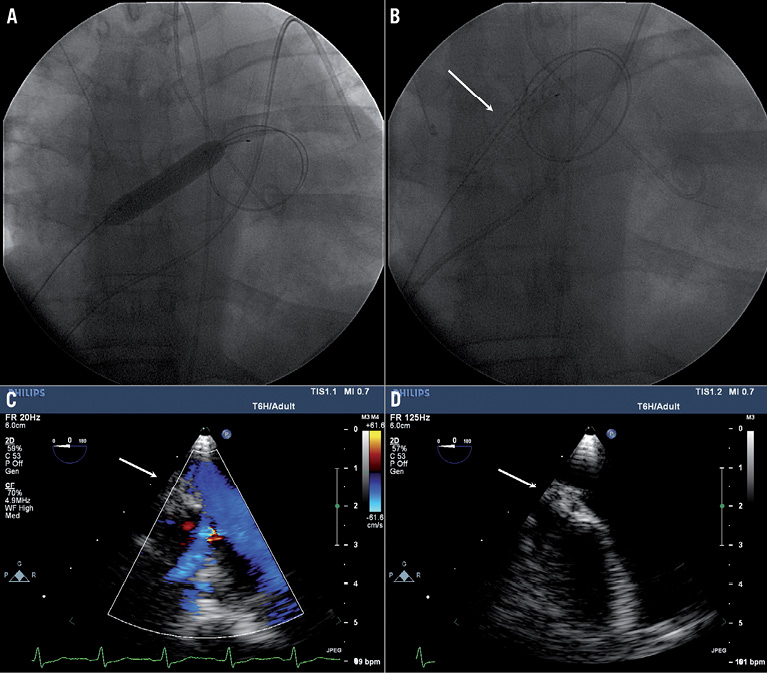
Figure 1. A) Atrial septostomy with a 10 mm× 4 cm balloon. B) Cryotherapy balloon septostomy with a 7 mm PolarCath (arrow). C) Transoesophageal echocardiogram 12 months after cryoballoon atrial septostomy demonstrates patency of the septostomy site. D) Honeycomb appearance of intra-atrial septum.
In this case, cryotherapy applied locally with the PolarCath during balloon atrial septostomy was associated with longer patency resulting in sustained improvement of symptoms as well as 6MWD, avoiding the need for reintervention. The follow-up TEE at one year post procedure revealed vacuolisation of the intra-atrial septum at the perimeter of the septostomy site. Whether these changes contributed to the lack of spontaneous closure is not known, nor is the long-term effect of these structural changes. The knowledge obtained based on one single case is limited and a meaningful conclusion cannot be drawn. The most important finding is that cryotherapy can be used safely during graded balloon atrial septostomy. Further studies will be needed to evaluate its safety and efficacy. Its future role in the treatment of patients with PH is uncertain, particularly when newer therapies such as riociguat and pulmonary denervation therapy are emerging.
Conflict of interest statement
The authors have no conflicts of interest to declare.

