
Chronic thromboembolic pulmonary hypertension (CTEPH) is an insidious disease and is associated with poor survival with medical therapy only1. The cumulative incidence of CTEPH following pulmonary embolism (PE) is 1% after six months, 3.1% after 12 months and 3.8% after two years after PE in anticoagulated patients2. While the extent of perfusion abnormalities following PE is a major determinant for the development of CTEPH, numerous studies have demonstrated that even large perfusion defects that do not resolve over time do not cause pulmonary hypertension, either at rest or on effort, in the majority of patients3. Only a subset of patients with large perfusion defects (or pneumonectomy) develops pulmonary hypertension over time, possibly due to the increased shear stress in regions with unobstructed pulmonary arteries. Indeed, lesions resembling those seen in idiopathic pulmonary arterial hypertension (PAH) are observed in CTEPH patients, including intimal thickening and remodelling of small pulmonary arteries, intimal fibrosis and fibromuscular proliferation as well as plexiform lesions4. Clinically, differentiation between idiopathic PAH and CTEPH may not be possible in patients with limited perfusion defects3. However, normalisation of pulmonary pressures is often achieved by pulmonary endarterectomy (PEA) or balloon pulmonary angioplasty (BPA), suggesting that treating the cause of the disease should remain the primary aim in CTEPH.
Contemporary management strategies significantly improve the disease trajectory. These include PEA, treatment with pulmonary vasodilators and BPA. PEA has been shown to improve survival significantly when compared to historical cohorts, with a significant improvement in haemodynamics, functional class, walking distance, and right ventricular function5. In large centres, perioperative mortality is relatively low (<5%), yet morbidity is substantial (including return to the operating theatre for any reason, re-intubation, tracheostomy, pneumothorax, intracranial bleeding or hospital stay of over two weeks), affecting up to 40% of patients6. Moreover, up to 40% of CTEPH patients are not suitable for PEA for a variety of reasons, including distal disease or significant comorbidity, which is common in CTEPH patients7. After PEA, up to 15% of patients suffer from residual pulmonary hypertension, which impacts on outcome8. Pulmonary vasodilators improve symptoms and reduce morbidity in patients with residual PH, but the net effect on haemodynamics appears rather modest.
Recent reports suggest that haemodynamic, symptomatic and prognostic benefits from BPA may be comparable to those of PEA. While currently reserved for patients who cannot (or will not) undergo PEA, BPA is evolving, as demonstrated by Shinkura et al9 in this edition of EuroIntervention.
The authors retrospectively compared two groups of patients undergoing BPA at their centre, all of whom achieved normalisation of their mean pulmonary arterial pressure (mPAP <25 mmHg) after treatment. Traditionally, BPA was performed until a normal mPAP was achieved, and no further procedures were pursued even when residual perfusion defects persisted (“conventional BPA group” in this study). However, in recent years the authors modified their BPA protocol, offering patients with normalised mPAP (baseline) additional BPA until all perfusion defects were treated (“extensive revascularisation BPA group” [ERBPA]). Comparison of the two groups demonstrated a significant improvement in haemodynamics (mPAP, pulmonary vascular resistance), exercise parameters (VE/VCO2 slope, six-minute walk test distance) and functional class with extensive revascularisation. There were no significant adverse events associated with the additional procedures in the ERBPA group.
This is potentially ground-breaking work, opening new avenues to BPA and the treatment of CTEPH: it seems that one should primarily aim at optimising pulmonary flow, regardless of pulmonary pressures, particularly in symptomatic patients. In a condition driven by chronic lung perfusion defects, the approach of treating the cause (perfusion defects) rather than the symptom (PH) does make sense, even when, after initial BPA treatment, CTEPH becomes chronic thromboembolic disease (CTED).
The potential merits of treating patients with CTED by PEA or BPA are not novel6,10. However, Shinkura et al are the first to demonstrate clearly an improvement in clinically relevant parameters in a large, well characterised cohort of patients. While the population described by Shinkura et al reverts to CTED from CTEPH after initial BPA treatment, their data support a “complete” or “extensive revascularisation” approach, avoiding CTEPH patients becoming symptomatic CTED patients.
The CTED population is significantly larger than that of CTEPH patients (Figure 1) and many suffer from exercise intolerance in the absence of PH. Hence, symptomatic patients with a history of PE but without features of obvious right ventricular pressure overload on echocardiography should be investigated further. Ventilation-perfusion (V/Q) scanning is instrumental in the diagnosis of CTED, and cardiopulmonary exercise testing has a role in quantifying exercise performance and investigating the contribution of CTED to breathlessness, even though data on this condition remain limited. Yet, the likelihood of establishing a diagnosis of CTED, especially in patients without a history of PE, remains low, and increased awareness of this condition is important.
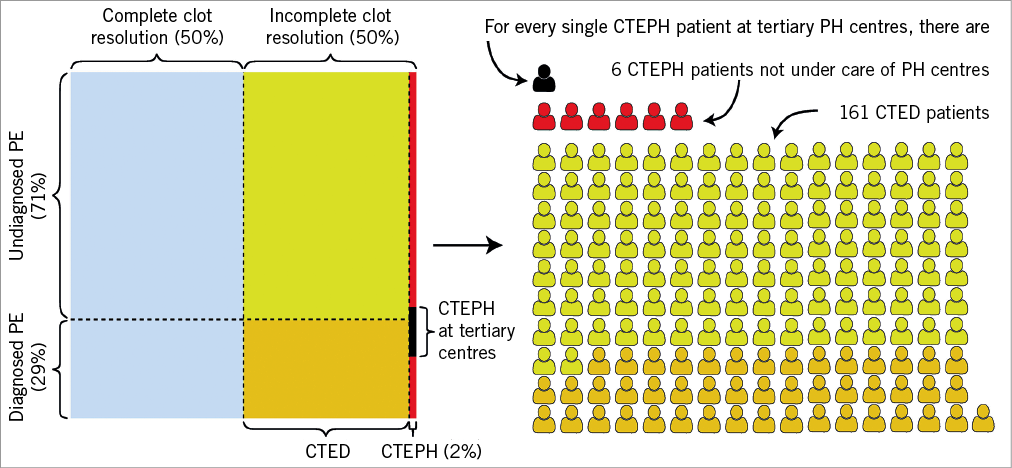
Figure 1. Estimated distribution of patients with pulmonary embolism and persistent pulmonary perfusion defects in the general population. Previous studies suggest that the true incidence of pulmonary embolism (PE) may be significantly higher than that observed, with a ratio of 2.5 cases of fatal PE diagnosed on autopsy (but not diagnosed prior to death), for every case of fatal PE diagnosed prior to death14; for the purpose of this Figure, this ratio has also been assumed in patients surviving PE. Furthermore, emboli resolve completely in less than 50% of PE patients15 (blue area). Patients with unresolved emboli develop either chronic thromboembolic disease (CTED) when mean pulmonary artery pressure is less than 25 mmHg, or chronic thromboembolic pulmonary hypertension (CTEPH) when pulmonary pressure is raised, with a conservative incidence estimate for CTEPH following PE of 2%. Patients with CTED and previously known PE are marked orange on the Figure, while those without PE history are marked yellow. Overall, 75% of CTEPH patients in national registries, which are based on data from tertiary pulmonary hypertension (PH) centres, have a history of PE. Based on these estimates and French registry data16, one can calculate that, for each CTEPH patient diagnosed in PH centres, there are six CTEPH patients in the community, and 161 CTED patients (114 without PE history), a subset of whom are likely to be symptomatic. The data from Shinkura et al, as well as prior authors, support extensive revascularisation in CTED patients and in patients in whom perfusion defects persist, after PEA or BPA, despite normalisation of pulmonary pressures. Further studies are urgently needed to establish the merits of this approach.
Hosokawa et al11, in this edition of EuroIntervention, use BPA to demonstrate the haemodynamic effects of unilateral treatment of CTEPH lesions on the untreated lung.
This study uses a combination of cardiac magnetic resonance imaging (CMR) and invasive measurements, and elegantly demonstrates that pulmonary blood flow increases in the treated lung but drops in the non-treated lung due to a reduction in PA pressure and, possibly, a “steal effect” by the treated lung. However, when using the data to calculate vascular resistance in each lung, there is a suggestion of a drop in pulmonary vascular resistance (PVR) in the non-treated lung, even though statistical significance is lost when repeated procedures in the same patient are excluded, in order to respect the assumption of independence of observations, on which the t-test relies. Despite this, the notion that relieving obstruction to flow in one lung benefits the other lung is intriguing and is in support of the complex pathogenesis and pathophysiology of CTEPH.
Interventional procedures have overtaken surgery in many areas of cardiovascular medicine. This is likely to become the case in CTEPH in the future, as demonstrated by the volumes of BPA cases performed in Japan and elsewhere. As the evidence base is expanding in favour of BPA and expected benefits appear to outweigh risks, BPA is likely to overtake PEA, with indications spanning from patients with distal CTEPH, patients not amenable to surgery, to symptomatic CTED patients, with a target of “extensive revascularisation”. Moreover, a hybrid PEA-BPA approach to patients with proximal and distal lesions, aimed at treating all perfusion defects independent of haemodynamics, should be investigated. While BPA does not require dedicated equipment and is currently successfully performed in many non-PEA centres around the world, expertise in the anatomy and pathophysiology of the pulmonary circulation and the management of CTEPH are essential for optimal outcomes. Using pressure wires to guide BPA may help to identify significant lesions that are not obvious on angiography but have a significant haemodynamic impact, hence improving the chances of achieving a more complete revascularisation12,13.
Efforts to increase the evidence behind this relatively novel and rapidly expanding procedure should continue, by establishing well-designed national and international registries, with close short- and long-term monitoring of patients.
Conflict of interest statement
A. Kempny, S. Wort and K. Dimopoulos have received educational or research grants from Bayer UK, GSK, Pfizer UK, Actelion UK and Actelion Global.

