Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) occurs as a consequence of a series of events that includes arterial obstruction by embolic material, secondary in situ thrombosis, cytokine activation and inflammation, and small vessel angiopathy. Medical therapies have a limited efficacy. Only the guanylate cyclase stimulator, riociguat, is approved for this condition. Surgical pulmonary endarterectomy is the definitive treatment for patients with proximal disease, but one third of patients with CTEPH are considered ineligible for surgery. Another third have significant residual pulmonary hypertension postoperatively. Balloon pulmonary angioplasty is an option for these patients. The procedure has a low procedural mortality and high efficacy in experienced centres but has not yet been subjected to rigorous evaluation in clinical trials. Alternative options for percutaneous management include atrial septostomy and pulmonary artery denervation. Experience with these procedures is accumulating, but adequately powered, controlled trials have not yet been performed.
Pathophysiology
When it is not acutely fatal, pulmonary thromboembolism is typically followed by gradual thrombus absorption and resolution without adverse sequelae. However, in a minority of affected individuals, estimated to be between 0.5 and 9.1%1, incomplete thrombus resolution and subsequent fibrous organisation result in mechanical obstruction of proximal and/or distal pulmonary arteries, leading to an increase in pulmonary vascular resistance and pulmonary hypertension. CTEPH may also occur in the absence of a history of acute pulmonary embolism or deep venous thrombosis2, either as a consequence of silent thromboembolism, or in situ thrombus formation in patients with thrombophilic disorders1,3. The direct impact of embolic material on pulmonary flow may also be compounded by a series of indirect effects including slow flow and secondary thrombus formation, inflammation, distal arterial vasoconstriction, and vascular remodelling due to endothelial and smooth muscle cell proliferation. This results in the formation of the plexiform lesions typical of other aetiologies of pulmonary hypertension2-6.
Angiographically, these changes are characterised by arterial stenoses or complete occlusion, web-like lesions, longitudinal bands, and the presence of bronchial arterial collaterals2,7-9. The condition is best diagnosed by a combination of ventilation/perfusion lung scanning, contrast CT imaging, and pulmonary angiography. Echocardiography and cardiac MRI are also valuable to document the effects of pulmonary hypertension on right ventricular size and function, and on the presence and severity of tricuspid valve regurgitation1.
Guideline-approved therapy
Without definitive therapy, the prognosis for patients with CTEPH and severe pulmonary hypertension (PH) is poor. In one series10, the five-year survival for male patients aged 40-50 years correlated strongly with the initial mean pulmonary artery (PA) pressure. Survival at five years of patients with a mean PA pressure of 41-50 mmHg was 35%, and of those with a mean PA pressure of >50 mmHg was 10%10. In another series, the three-year survival was only 10% in patients with a mean PA pressure of >30 mmHg11.
Although medical therapy with endothelin receptor antagonists, phosphodiesterase-5 inhibitors and prostacyclin analogues has been used to treat patients with CTEPH1, none of these has been definitively shown to provide benefit in the CTEPH population. In some series12, bridging therapy prior to surgery was associated with a less favourable outcome than in patients who did not receive medical therapy. The only medication currently approved for the treatment of inoperable CTEPH is the novel compound riociguat, a soluble guanylate cyclase stimulator13.
Pulmonary endarterectomy (PEA) is considered the definitive therapy and is potentially curative1,14,15. The procedure involves meticulous surgical removal of all visible thrombus and distal fibrotic material in segmental and subsegmental arteries. Although high surgical mortality rates have been reported in the past2, the perioperative mortality of surgical endarterectomy has fallen and is now reported to be <10% in experienced centres14,16. Five-year survival after successful surgery has also improved and now approaches 90%12,16. The surgery is complex and demanding, and has been limited to a small number of centres of excellence in many countries.
Balloon pulmonary angioplasty
Although progress in the surgical management of CTEPH has been made unequivocally over the past decade, many patients with CTEPH are considered ineligible for endarterectomy because of surgically inaccessible distal vessel disease (Figure 1), advanced age or comorbidities. Others have significant residual postoperative PH. In the series of Condliffe and colleagues16, 32% of patients evaluated for surgery were considered inoperable. In the same series, 35% of the patients who survived surgery had persistent postoperative pulmonary hypertension16.
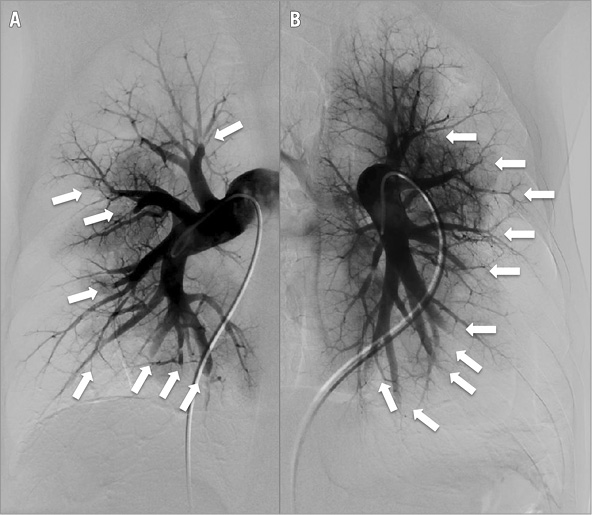
Figure 1. Digital subtraction angiography (DSA) of the right (A) and left (B) pulmonary arteries showing multiple lesions (arrowed) in CTEPH.
For these patients, balloon pulmonary angioplasty (BPA) has been proposed as an attractive and effective option (Figure 2). The procedure is based on techniques developed for the management of congenital pulmonary artery stenosis17,18. In early experience, low-pressure balloon inflation was performed with a balloon to artery ratio of 2:1-3:117, but the technique was later modified to use smaller diameter balloons (balloon to artery ratio <2:1) at higher pressure18. The first series of patients with CTEPH treated by BPA was reported by Feinstein and colleagues in 200119. The procedure was complicated by reperfusion pulmonary oedema in 11 of the 18 patients (61%) but resulted in a significant reduction in mean PA pressure, with an associated improvement in NYHA class and six-minute walk time (6MWT) at three months19.
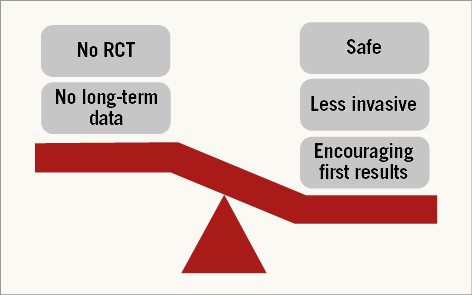
Figure 2. Pros and cons of balloon pulmonary angioplasty for CTEPH. RCT: randomised controlled trial.
Since the initial reports, experience with BPA has accumulated, particularly in Japan and subsequently elsewhere20,21. In selected patients, the improvement in haemodynamic parameters and clinical outcome following successful BPA may be comparable to that of PEA22. Published series have confirmed the observations of Feinstein with a significant reduction in mean PA pressure and pulmonary vascular resistance (PVR), an increase in cardiac output and arterial oxygen saturation, a reduction in B-natriuretic peptide (BNP), and improvement in functional class23-27. The study of Fukui and colleagues25 also showed a favourable effect of BPA on right ventricular (RV) remodelling, measured by cardiac magnetic resonance (MR) imaging three to six months post-procedurally, with a significant reduction in RV volume and RV mass index, and a rise in RV ejection fraction (Figure 3). The changes in RV performance correlated strongly with the change in pulmonary haemodynamic parameters25. Similar changes have also been documented by echocardiography after BPA26, and following PEA by cardiac MR28. Importantly, in follow-up studies, the acute haemodynamic and functional benefits of BPA seem durable, at least to one-year follow-up, with a negligible incidence of recurrent PA stenosis20,23.
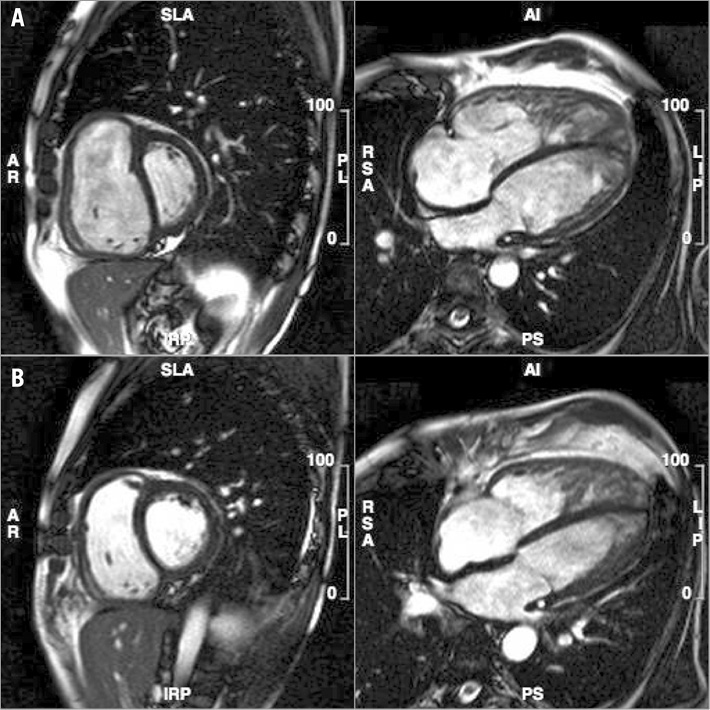
Figure 3. Cardiac MRI before (A) and after (B) BPA demonstrating right ventricular remodelling.
Histologic and imaging studies pre- and post-BPA have shown a variety of pathologies and various mechanisms of balloon dilatation of pulmonary stenoses29-31. Pre-BPA OCT imaging has shown lesions composed of lipid-rich plaques, organised thrombus, and honeycomb structures that can be difficult to identify angiographically29. BPA results in medial dissection and compression of organised thrombus and microchannels31, and breakdown or disruption of the webs and honeycomb lesions30,32.
The procedure has continued to evolve with changes implemented to minimise the risk of complications. In particular, multiple strategies have been described to reduce the risk of reperfusion lung injury and alveolar oedema, and the associated risk of respiratory failure22,23,30,32-34. The phenomenon has been well studied in patients treated by PEA. In an early series of 22 patients who underwent PEA at UC San Diego between 1970 and 1984, radiological abnormalities were identified in all but one patient35. Pulmonary infiltrates developed within 72 hrs and persisted for a mean of nine days. The most common radiological appearance was “focal pulmonary oedema”, which occurred only in the distribution of treated artery segments. In each patient, the left atrial pressure or pulmonary capillary wedge pressure was normal (<14 mmHg)35. The authors speculated that the mechanisms of the non-hydrostatic pulmonary oedema could include tissue injury mediated by the release of oxygen free radicals or inflammatory cytokines, and a possible role for the extensive bronchial collateral circulation seen in this condition35.
Following BPA, reperfusion lung injury occurs with a similar frequency to post PEA22,34. Several predictors of the complication have been identified. In one study, the risk of reperfusion injury was related to the baseline pulmonary pressure, with an increased risk in patients with a pre-intervention mean PA pressure >35 mmHg19. In a study by Inami and colleagues33, baseline mean PA pressure, and baseline PVR were independent predictors by multivariate analysis, but the strongest correlate was a novel index, the PEPSI (Pulmonary Edema Predictive Scoring Index) score, that incorporates the change in segmental pulmonary blood flow and the baseline PVR. The number of arteries treated per session was not predictive33. The practical consequence of this finding is that, in patients with a very high PA pressure and PVR, the size of the territory revascularised matters more than the number of vessels treated. For example, when a large arterial segment is affected (e.g., the basal segments 8, 9 or 10), BPA should be limited to one subsegment to reduce the risk of reperfusion injury and alveolar oedema. If required, additional segments should be treated in staged procedures.
Based on these observations, the technique of BPA for CTEPH has been modified in many centres, using guidance by sequential physiological assessment or intravascular imaging. Inami and colleagues used a 0.014” pressure guidewire to guide balloon inflations, with the aim of achieving a distal pressure to proximal pressure ratio >0.822. Further balloon inflations of each stenosis were stopped when the mean distal pressure rose to 35 mmHg. The authors retrospectively compared cohorts of consecutive patients treated without pressure wire guidance (group 1), with pressure wire guidance alone (group 2), and with guidance using both the PEPSI score and physiological assessment (group 3)22. The more intensively guided group of patients (group 3) had a significantly lower incidence of moderately severe or severe reperfusion lung injury, with the same haemodynamic improvement in fewer treatment sessions22.
Other approaches to minimising complications of BPA have included intravascular ultrasound imaging23,34 and OCT30,32. In these studies, maximum balloon diameter was limited, usually to <90% of the PA diameter, to minimise the risk of arterial dissection and perforation.
Patient selection for BPA
BPA is listed in the current guidelines for the treatment of PH as an option for patients who are considered inoperable, or who have an unfavourable risk:benefit ratio for PEA as a class IIb recommendation with a level of evidence C1. Decisions made regarding suitability for surgery versus angioplasty should be made at expert referral centres by a multidisciplinary team which includes cardiologists with expertise in the medical management of pulmonary hypertension, respiratory physicians, cardiac surgeons with expertise in PEA, radiologists and other imaging specialists, intensive care specialists and/or anaesthetists, a clinical nurse specialist, and social work/geriatricians. The BPA procedure should be performed by an experienced interventional radiologist or interventional cardiologist. The institution should have appropriate facilities for postoperative management including extracorporeal membrane oxygenation (ECMO) therapy and, ideally, lung transplantation.
Patients considered for BPA should have severe PH (mean PA ≥25 mmHg), and severe PVR (≥3 Woods units or 240 dynes·sec·cm–5), should be symptomatic (NYHA or WHO functional class ≥II), and should be considered poor candidates for PEA. Anatomical features that would favour BPA include focal stenoses, webs, bands, intimal irregularities, abrupt narrowings, and some chronic total occlusions in segmental or subsegmental pulmonary arteries that are not readily accessible surgically20. Optimal medical therapy should be instituted prior to intervention using oral anticoagulants and appropriate anti-failure therapy. In some patients, angiographic assessment may suggest operable disease in one lung, and inoperable disease on the contralateral side. In this situation, consideration should be given to a hybrid approach, treating the inoperable disease by BPA first or at the time of the pulmonary endarterectomy (Figure 4)36.
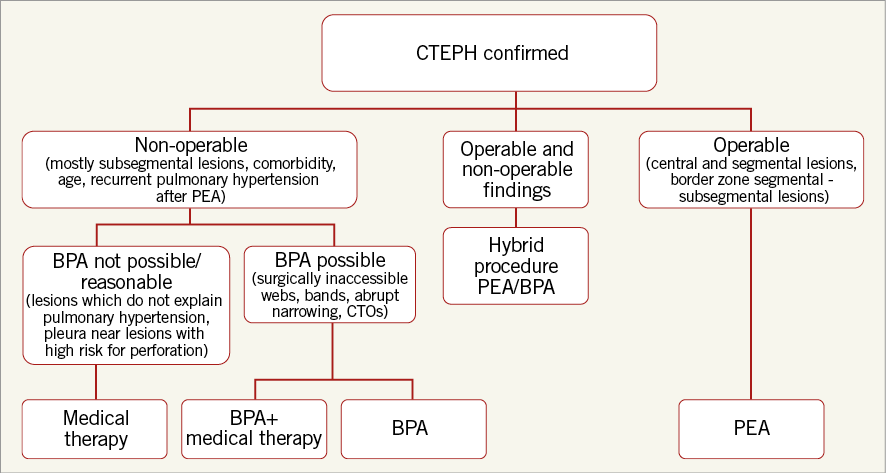
Figure 4. Decision tree for management of CTEPH. BPA: balloon pulmonary angioplasty; CTO: chronic total occlusion; PEA: pulmonary endarterectomy
BPA procedure
BPA is best performed as a staged procedure, often as a series of three to five sessions over a period of several weeks to minimise the risk of reperfusion injury. Heavy sedation should be avoided since wiring of the target lesion often requires deep inspiration and breath holding. Access is obtained from either the right internal jugular vein or right femoral vein. Access to the right pulmonary artery may be easier in some arterial anatomies using an internal jugular approach but, in experienced hands, both pulmonary circulations can be accessed from a femoral approach. Once access is obtained and anticoagulant therapy given, a 6 Fr introducer sheath is advanced into the main pulmonary artery, or right or left PA over a 0.035” guidewire. A 90 cm sheath is used for femoral access, and an 80 cm sheath is used from the internal jugular vein. Through the sheath, a 6 Fr guiding catheter (Multipurpose or JR4) is advanced to the arterial segment to be treated. The lesion is crossed with a 0.014” wire, sometimes interrogated with IVUS or OCT, and dilated with undersized, rapid exchange balloons (Figure 5, Figure 6). Inflations are performed sequentially aiming to restore flow in the arterial and venous circulations, and reduce stenosis severity without necessarily attempting to eliminate the arterial narrowing completely (Figure 5). Several lesions can be treated in one session, though the treated lesions are often limited to one or two pulmonary segments. Since the lower lobes have a more extensive vascular supply than the upper or middle lobes, lesions in the lower lobes are commonly treated first. Some operators prefer to limit procedural time, radiation dose and contrast dose rather than the number or distribution of lesions treated20,22,25. Three-dimensional rotational angiography with image overlay has been used in some centres to provide roadmap guidance to select projection angles, and reduce fluoro time and contrast dose.
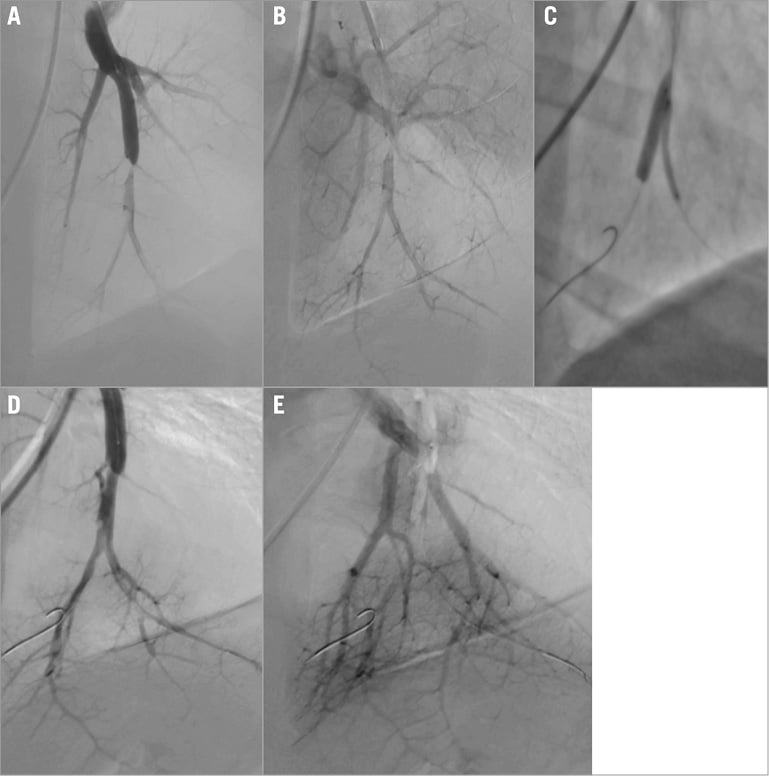
Figure 5. BPA procedure for bifurcation disease. A) Selective DSA showing subtotal peripheral occlusion. B) Venous run-off before BPA. C) Kissing balloon BPA of the bifurcation lesion. D) Selective DSA post-BPA. E) Venous run-off after BPA.
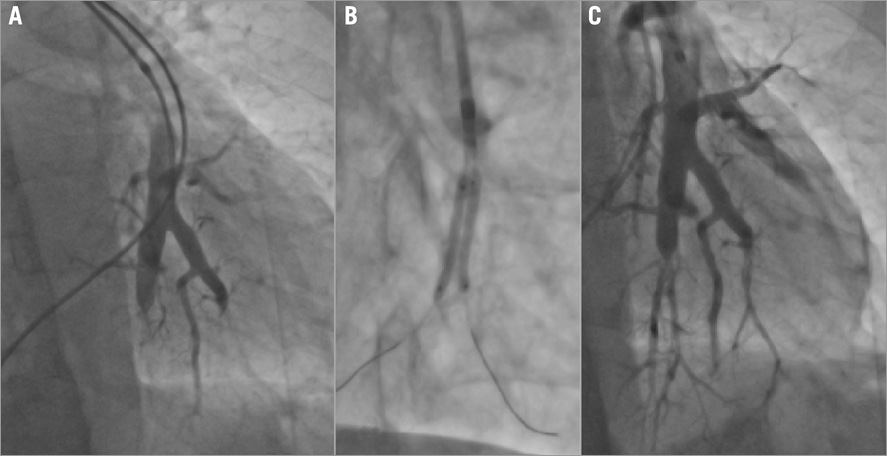
Figure 6. Chronic total occlusion (CTO) and bifurcation disease. A) Selective angiogram showing chronic occlusions. B) BPA with two 2.0×20 mm compliant balloons. C) Final result after BPA of recanalisation of two CTOs.
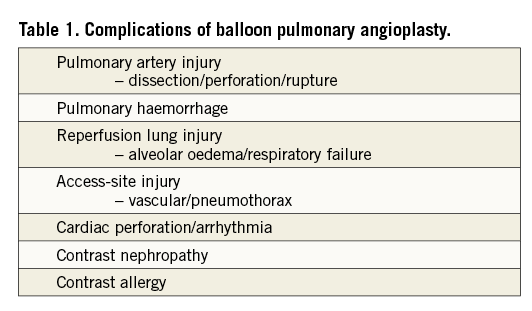
Complications of the procedure other than reperfusion lung injury are relatively infrequent. They include pulmonary artery dissection, perforation or rupture, cardiac perforation, arrhythmias, pulmonary haemorrhage and haemoptysis, contrast allergy and contrast-related renal injury, and access-site injury including pneumothorax (Table 1).
Atrial septostomy
In selected patients with PH, the creation of a right to left shunt by atrial septostomy has provided symptom relief and objective improvement in exercise performance. Although this has most frequently been used for treatment of other causes of PH37, it could be considered an option in patients with inoperable CTEPH who are not good candidates for BPA, and in those with persisting post-PEA PH. The technique is based on the observation that patients with severe PH associated with Eisenmenger’s syndrome have a more favourable haemodynamic profile, better functional capacity and a better prognosis than patients with primary PH38. The presence of a shunt is thought to increase left ventricular pre-load and cardiac output, decompress the right atrium and ventricle, and reduce sympathetic overactivity. Although systemic arterial oxygen saturation falls, systemic oxygen transport rises. This has been shown to improve right ventricular performance39, reduce B-type natriuretic peptide (BNP) levels40, and reduce muscle sympathetic nerve activity (MSNA)41.
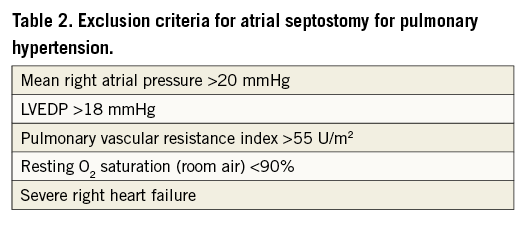
The technique of atrial septostomy has been described in detail elsewhere42. Originally conceived as a palliative treatment for infants born with cyanotic congenital heart disease (specifically complete transposition of the great arteries) by William Rashkind in 196643, the procedure was first described as a treatment for PH almost two decades later37. Early experience with blade septostomy44 and subsequently with graded or stepwise balloon dilatation45 was associated with a high procedural mortality. Based on this experience, recommendations for minimising procedural mortality were proposed39. These included exclusion of patients with severe right heart failure, a mean right atrial pressure >20 mmHg, an indexed PVRI >55 U/m2, resting arterial O2 saturation <90% on room air, and LVEDP >18 mmHg39,46 (Table 2). With these guidelines, the procedural mortality rate has been considerably lower with rates of 2-5% reported in some series39,47.
Alternatives to balloon septostomy for creating a right to left shunt at the atrial level have also been described42. These include the use of septal devices to improve the predictability and durability of the treatment effect. Their role remains uncertain at this time.
Pulmonary artery denervation
Like atrial septostomy, pulmonary artery denervation (PADN) is a novel procedure being used predominantly as a treatment for primary PH42. However, it could also be considered for patients who are inoperable, or who have residual PH after PEA or BPA48. Patients with PH have increased sympathetic nervous system activity as measured by microneurography, and right ventricular β-adrenergic receptor downregulation has been demonstrated in patients with PH and right heart failure42. Stretch receptors in the pulmonary artery may also cause vasoconstriction, adding to the increased PVR42. The potential for PADN as a treatment for PH has been evaluated in preclinical models. In a canine model of PH, Zhou and colleagues49 showed that PADN caused neural demyelination, axon loss and impaired conduction velocity in pulmonary sympathetic nerves. This was associated with substantial reductions in mean PA pressure, PVR, and medial wall thickness compared to a sham treated group49. It should be noted that the canine model is not an ideal model for human pulmonary hypertension, and has not been predictive of the human response to medical therapies.
Nonetheless, in an uncontrolled series of 66 patients, nine of whom had CTEPH, Chen and colleagues48 showed an improved functional class and 6MWD, and reduced pro-BNP levels 12 months after PADN therapy. Clearly this will need to be further evaluated in larger, controlled trials before being incorporated into the list of therapeutic strategies for CTEPH.
Conclusions
Untreated, CTEPH is associated with substantial morbidity and a high mortality. In experienced centres, the prognosis for patients with surgically accessible disease has been improved considerably by PEA. Patients with surgically inoperable disease, and with persisting PH after endarterectomy, may be candidates for BPA. The technique has been refined progressively in recent years to include physiological and intravascular imaging guidance. To date, large-scale controlled clinical trials have not yet been performed but the experience reported from small series is encouraging. It is too early to speculate on whether BPA may eventually become a first-line therapy for surgically operable disease, but its use in high-risk individuals is likely to increase. For the foreseeable future, patients with central or segmental disease should be treated surgically. When the disease predominantly affects subsegmental arteries, balloon angioplasty is usually preferred. Other approaches to managing CTEPH percutaneously remain investigational.
Conflict of interest statement
The authors have no conflicts of interest to declare.

