Abstract
Aims: To evaluate the efficacy and safety of balloon pulmonary angioplasty (BPA) in patients with non-operable chronic thromboembolic pulmonary hypertension (CTEPH) using the results of pulmonary endarterectomy (PEA) for operable patients as a reference, and annotate the role of BPA in the management of CTEPH.
Methods and results: Data from 53 CTEPH patients were collected retrospectively. Twenty-four operable patients underwent PEA, and 29 non-operable patients underwent BPA. Patients who underwent BPA showed improved mean pulmonary arterial pressure, pulmonary vascular resistance, and cardiac output (39.4±6.9 to 21.3±5.6 mmHg, 763±308 to 284±128 dyn·s–1·cm–5, 3.47±0.80 to 4.26±1.15 L/min, respectively); patients who received PEA showed similar efficacy (44.4±11.0 to 21.6±6.7 mmHg, 781±278 to 258±125 dyn·s–1·cm–5, 3.35±1.11 to 4.44±1.58 L/min, respectively). The mortality rates of BPA and PEA patients were 3.4% and 8.3%, respectively.
Conclusions: The efficacy and safety of BPA for non-operable cases were similar to those achieved using PEA for operable cases. BPA could be an additional treatment option for non-operable CTEPH patients, and most CTEPH patients can be satisfactorily treated by BPA or PEA.
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterised by stenosis and obstruction of the pulmonary artery (PA) with non-resolving organised thromboemboli, leading to elevated pulmonary vascular resistance (PVR), severe pulmonary hypertension (PH), right heart failure, and finally death1-3. Without treatment, the prognosis for patients with CTEPH is very poor, with a five-year survival rate of 10% in patients with a mean PA pressure (mPAP) >50 mmHg4. Pulmonary endarterectomy (PEA) is the gold standard for the treatment of CTEPH; however, up to 40% of CTEPH patients are judged non-operable owing to distal thromboembolism or comorbidities5. Although targeted medical therapy established for pulmonary arterial hypertension6 may be effective in non-operable patients, its efficacy is limited and the associated mortality rate remains high7-9. Currently, no established treatment strategies improve the prognosis of patients with non-operable CTEPH.
In 2001, Feinstein et al reported that balloon pulmonary angioplasty (BPA) significantly improved symptoms and haemodynamics in non-operable CTEPH patients10; however, the efficacy and safety were not satisfactory. Recently, with refinements in the technique, some groups have demonstrated the efficacy and safety of BPA in non-operable patients11-13. BPA may become an alternative treatment strategy for non-operable patients; however, it is an emerging therapy, and there is no consensus about the role of BPA in the management of CTEPH.
The purpose of this study was to evaluate the efficacy and safety of BPA for non-operable patients, utilising the results obtained using PEA for operable patients as a reference. Additionally, we sought to examine the role and the indication for BPA as a treatment strategy for CTEPH. We did not attempt to discuss the differences in clinical efficacies between BPA and PEA, because the lesions targeted by BPA and PEA are different. We aimed to comment on the roles of BPA and PEA in the management of patients with CTEPH.
Methods
STUDY DESIGN
Between September 2001 and September 2013, a total of 59 patients were diagnosed with CTEPH at Kobe University Hospital. The diagnosis of CTEPH was established according to clinical guidelines6, based on medical history, physical examination, chest radiography, echocardiography, computed tomography (CT) scan, lung ventilation-perfusion scintigraphy, right heart catheterisation (RHC), and pulmonary angiography (PAG). Six patients who did not consent to therapeutic intervention were excluded from the study. Finally, 53 patients with CTEPH were reviewed retrospectively.
From March 2011, our institute started performing BPA for non-operable patients. Between September 2001 and March 2011, 31 patients were diagnosed with CTEPH. Of these, 17 patients judged as operable underwent PEA, while 14 patients judged as non-operable were treated medically. From March 2011, 22 more patients were diagnosed as having CTEPH. Of these, seven patients underwent PEA and 15 underwent BPA. In total, 29 patients underwent BPA, including the 14 patients diagnosed before March 2011, who received medical treatment, plus 15 of the patients newly diagnosed with CTEPH.
ASSESSMENT OF OPERABILITY
The assessment of operability was conducted by cardiologists and cardiovascular surgeons. The decision regarding operability was based on the criteria for PEA14. The distribution of thromboembolic lesions and intimal thickening of PA was evaluated using chest CT and PAG, and reviewed by cardiologists, cardiovascular surgeons, and radiologists. The main responsible lesions in the main trunk and those from the lobar to the segmental artery were defined as proximal, while the distal segmental to subsegmental lesions were defined as distal. Surgical accessibility to lesions was decided after consensus between experienced cardiovascular surgeons and cardiologists, and for this several factors were considered, including lesion distribution, intimal thickening at the lesions, and patient age that could affect the fragility of intima. Patients judged as operable underwent PEA, and those judged as non-operable were treated medically for at least three months and then underwent BPA. Patients provided written informed consent to undergo PEA or BPA.
BPA procedure
BPA was performed using techniques similar to those previously described11. We approached the PA through the right femoral (82.6%) or jugular (17.4%) vein using a 6 Fr long sheath (BRITE TIP® Interventional Sheath Introducer; Cordis/Johnson & Johnson, Bridgewater, NJ, USA) which was introduced via a 9 Fr sheath (Arrow-Flex®; Teleflex Medical, Durham, NC, USA). Heparin (5,000 U) was administered after the placement of the 9 Fr sheath and was added every hour to maintain an activated clotting time between 200 and 300 s. A 6 Fr guide catheter (Autobahn; Multipurpose, or Judkins right 4.0; NIPRO Corporation, Osaka, Japan) was inserted through the long sheath and was advanced to the target vessels. Based on PAG, a 0.014-inch guidewire (Cruise; Asahi Intecc, Tokyo, Japan) was passed across the target lesion. While selecting the target vessels, the lower lobe lesions were preferentially dilated because the pulmonary blood flow at this site was relatively high, thus lowering mPAP. The lumen diameter was measured using intravascular ultrasound (IVUS) (Eagle Eye® Platinum; Volcano Corp, Rancho Cordova, CA, USA). IVUS was also used to check whether the guidewire had crossed eccentrically to ensure that the PA had not ruptured while ballooning. In cases of severe stenosis, abrupt narrowing, or complete obstruction, initially a 2.0 mm balloon catheter was used to dilate the lesions. Subsequently, the lesions were dilated to an appropriate size using 2.0 mm to 9.0 mm balloon catheters depending on the diameters of the vessels (Bandicoot RX; St. Jude Medical, St. Paul, MN, USA; Shiden; Kaneka Medix, Osaka, Japan; Sterling Monorail®; Boston Scientific, Natick, MA, USA). To prevent PA injury, the maximum balloon size was slightly smaller than the actual vessel diameter. We treated one to six segmental to subsegmental arteries in each procedure according to mPAP, which is the main indicator of reperfusion pulmonary injury (RPI)15. The procedure was repeated twice within one to two weeks in the course of one admission. BPA was performed for a total of one to six sessions to normalise mPAP. We used non-invasive positive pressure ventilation (NIPPV) to prevent RPI in the first 50 sessions at least 24 hours after BPA. In the last 36 sessions, NIPPV was used only if needed. Epoprostenol was used in some cases if mPAP was over 40 mmHg, and dobutamine was administered if the cardiac index was <2.0 L/min/m2 up to three days after BPA. A chest CT was performed within six hours after BPA to evaluate RPI, which was indicated as a high-density area of the dilated segments. Haemodynamic data were obtained in all patients around seven days after BPA.
PEA procedure
PEA was performed using techniques similar to those established by the San Diego group16,17. Bilateral PEA was performed through a median sternotomy. Distal endarterectomy was conducted with intermittent circulatory arrest for a period limited to 20 minutes, while the central temperature was maintained at 16°C. Patients were provided with perioperative medical treatment using catecholamines, PDE3 inhibitors, epoprostenol or diuretics until recovery. We classified lesions based on the location and morphology at the time of operation, as previously defined18. Haemodynamic data were obtained around two weeks after PEA. Residual PH after PEA was defined as mPAP >25 mmHg.
Statistical analysis
Quantitative values are presented as mean±standard deviation. Differences in values were tested using the Student’s t-test and the paired Student’s t-test. Nominal data were expressed as numbers and percentages and were analysed using the χ2 test. World Health Organization functional class (WHO-Fc) was presented as the median and number of patients in each class, and the differences were tested by the Mann-Whitney test. The level of statistical significance was set at p<0.05. All statistical analyses were performed using GraphPad Prism version 5 (GraphPad Software, La Jolla, CA, USA) and SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA).
Results
CLINICAL CHARACTERISTICS
The baseline characteristics of the study population are shown in Table 1. The mean age was significantly higher in the BPA group than in the PEA group. The median time from diagnosis to PEA or BPA was 113 days or 802 days, respectively. Most patients judged as non-operable had only distal lesions. Vasodilators were administered to 29.2% of patients who underwent PEA and 82.8% of patients who underwent BPA. Table 2 shows the main reasons patients were judged as non-operable. We abandoned PEA and performed BPA in four patients who had proximal segmental lesions, because of severe COPD, the co-existence of cancer, advanced age, and a history of stroke.
PROCEDURES AND OUTCOMES OF BPA AND PEA
A total of 86 BPA sessions were performed in 29 patients (average 2.97 sessions per patient). The number of dilated vessels was 2.74 per session. All distal segmental and subsegmental lesions were accessible. The average balloon size used in BPA was 4.35±1.78 mm. The required contrast medium was 195±64 mL per session. Perioperative medical treatment with catecholamines or epoprostenol was performed in 21 sessions (24.4%). The median stay in intensive care unit after BPA was 2.96±1.59 days. Figure 1 and Moving image 1-Moving image 6 show representative PAGs, procedures, and IVUS footage of BPA. Blood flow of the PA improved dramatically after BPA.
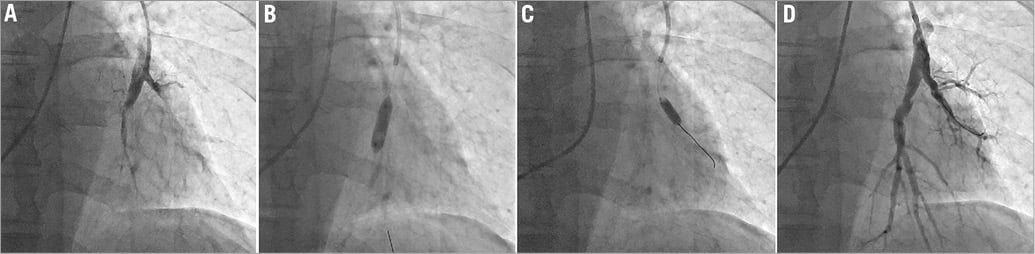
Figure 1. Representative pulmonary angiograms of the segmental pulmonary artery (left A10) from a patient who underwent BPA: before BPA (A) and after BPA (D). A pulmonary artery with abrupt narrowing is effectively dilated by a 5 mm balloon at 8 atm (B, C). BPA: balloon pulmonary angioplasty
A total of 24 patients underwent PEA. The mean duration of circulatory arrest, cardiopulmonary bypass, and surgery were 47.0±24.67 min, 235.9±44.3 min, and 424±151 min, respectively. An inferior vena cava filter was placed in 12 patients (50.0%).
Perioperative medical treatment with catecholamines, PDE3 inhibitors, or epoprostenol was performed in 21 patients (87.5%).The mean duration of mechanical ventilation was 2.3±1.8 days, and the median stay in intensive care unit after PEA was 8.67±2.87 days. Intraoperative findings showed that 58.3% had surgical classification types I and II, and 41.7% had type III. There were no patients with type IV lesions. Figure 2 shows representative PAG images before and after PEA and a thromboembolic specimen from the same patient.
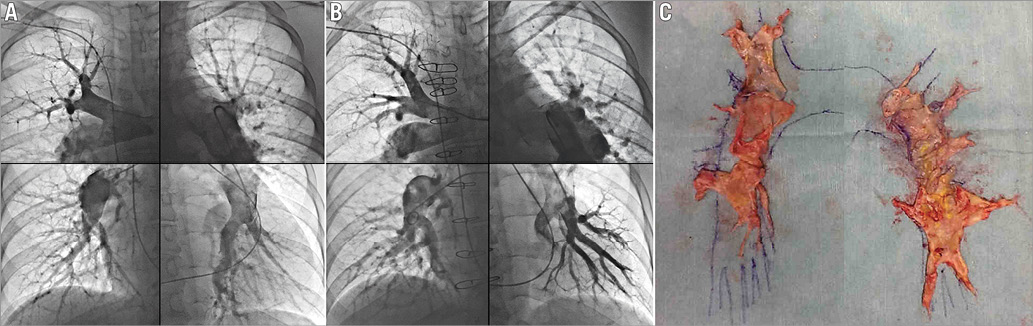
Figure 2. Representative pulmonary angiograms from an operable patient before (A) and after (B) PEA, including each part of the lung. Thromboembolic specimen removed from the same patient (C). The disease type is Type III on both sides. PEA: pulmonary endarterectomy
Although medical vasodilator therapies did not significantly improve haemodynamics and symptoms in the BPA group, BPA dramatically improved the patients’ haemodynamics and clinical status to a similar degree to PEA (Table 3, Figure 3). Exercise capacity and haemodynamics in both operable and non-operable patients improved remarkably after treatment with BPA or PEA.
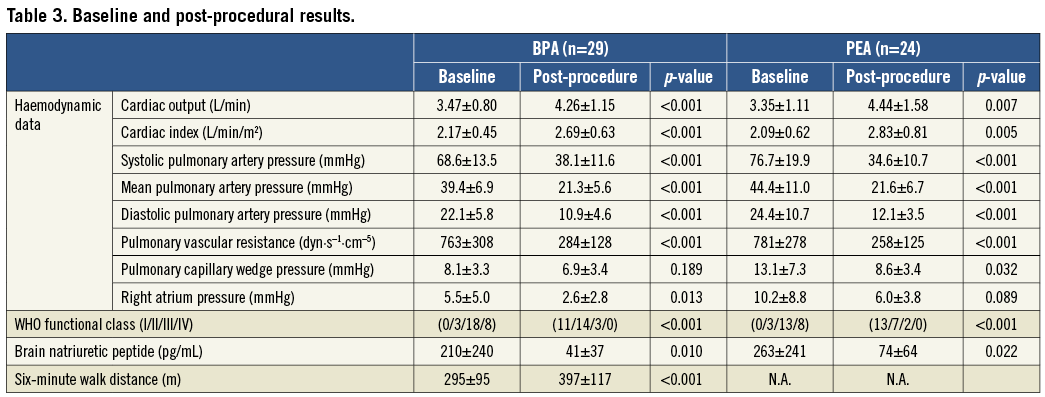
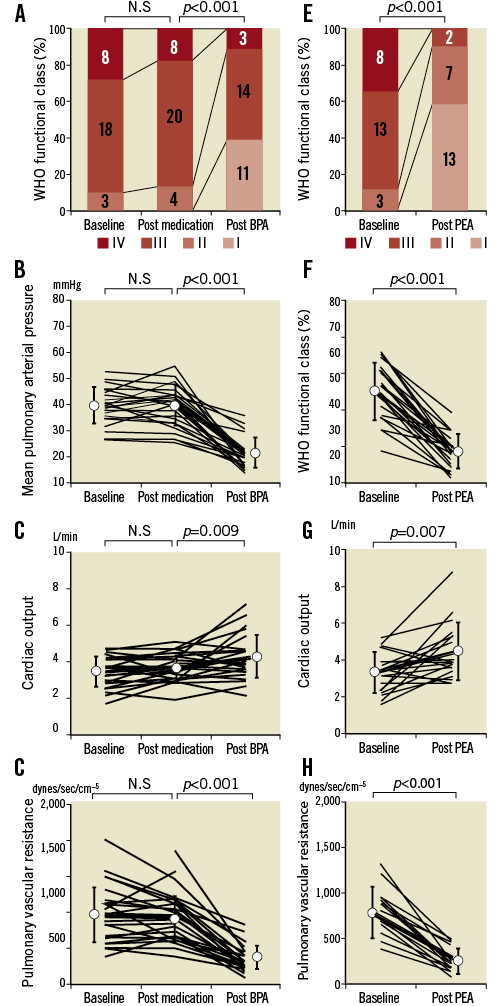
Figure 3. Changes in WHO functional class and haemodynamics before and after vasodilator therapy and after balloon pulmonary angioplasty (A-D) and pulmonary endarterectomy (E-H). WHO functional class (A, D), mean pulmonary arterial pressure (B, F), cardiac output (C, G), and pulmonary vascular resistance (D, H). WHO: World Health Organization
COMPLICATIONS OF BPA AND PEA
Complications due to BPA and PEA are presented in Table 4. RPI was the main complication of BPA and occurred in 64.0% of sessions, but 50.9% of the RPI cases were asymptomatic. Symptomatic RPI was associated with haemoptysis or haemoglobin desaturation. These patients required additional oxygen therapy, NIPPV, or intratracheal intubation. The use of NIPPV decreased to 58.1% in the more recent half of the sessions, that is, between September 2012 and September 2013, because it was not always necessary. Furthermore intratracheal intubation was not required in these patients. In case of wire perforation, we could control bleeding in all cases by ballooning the vessel at the proximal edge under low pressure. Wire perforation also decreased as the operators became more skilled in conducting the procedures. None of the patients required cardiopulmonary support. There was one in-hospital death related to infection resulting from central venous catheter use for epoprostenol administration.
Among those who underwent PEA, half of them had operative complications, including RPI, persistent PH, infection, and neurological complications. Two patients died 16 days and five days after PEA. Both patients had severe persistent PH and low cardiac output (CO) and required constant cardiopulmonary support. The in-hospital mortality rate of PEA was lower in cases reported between November 2006 and September 2013, and there was no in-hospital death among these patients.
Follow-up
In the BPA group, there was one death from malignant cancer during the follow-up for 1.15±0.81 years after the final sessions, and one in-hospital death as described above. None of the remaining 27 patients had re-exacerbation of symptoms. Twenty-three patients underwent RHC at 0.92±0.52 years after the final sessions and had mPAP of 22.5±5.7 mmHg, CO of 4.26±0.91 L/min, and PVR of 291±157 dyn·s–1·cm–5. The estimated right ventricular systolic pressure (RVSP) of 25 patients from follow-up echocardiography at 1.10±0.59 years remained almost equivalent from 38.4±8.5 mmHg to 37.2±10.1 mmHg. After the final BPA, the dose of medical therapy was reduced or was discontinued in seven patients (29.1%).
In the PEA group, all patients were alive during the follow-up for 4.69±3.60 years, except for two patients who had died perioperatively. Recently, five patients underwent follow-up RHC at 1.12±0.64 years after the PEA and had mPAP of 17.6±5.3 mmHg, CO of 3.86±0.41 L/min, PVR of 255±162 dyn·s–1·cm–5. The RVSP of 18 patients after 3.93±3.57 years of follow-up was unchanged from 29.8±8.8 to 30.9±6.6 mmHg. One patient who had re-exacerbation of symptoms and pulmonary hypertension underwent BPA. Figure 4 shows the survival curve of the BPA group and the PEA group by the Kaplan-Meier method.
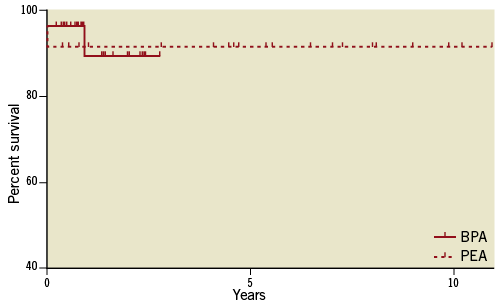
Figure 4. The survival curve of the BPA group and the PEA group by the Kaplan-Meier method from the last procedures. Log-rank test, p=0.92.
Discussion
This is the first study to describe the efficacy and safety of BPA for patients with non-operable CTEPH using the efficacy and safety achieved by PEA for operable patients as reference. BPA could effectively improve haemodynamics and exercise capacity in non-operable patients who could not get benefit with medical therapy, similar to those of PEA in operable patients. With regard to complications, the mortality rate of BPA was comparable to that in previous reports11,19 and was not higher than that with PEA in this study or in other experienced centres20. The frequency of RPI with BPA and PEA was also comparable to those reported previously11,21. In BPA, only a few cases required intratracheal intubation and had a few other complications. Thus, although it cannot necessarily be said that BPA was a minimally invasive therapy, the efficacy and safety of BPA for non-operable patients were as satisfactory as those of PEA for operable patients. These results suggest that most CTEPH patients can be treated successfully with either BPA or PEA.
To decide the treatment strategy for CTEPH patients, it is most important to assess surgical operability; however, there is no universal consensus regarding operability. The indications for non-operability vary from 12% to 60.9% among different centres and countries5.
Comorbidities are one of the main factors influencing the decision. COPD is a frequent comorbidity observed in 9.5% of CTEPH patients5, and severe chronic lung disease is a contraindication for PEA. In the present study, two patients who were judged as non-operable owing to severe COPD were successfully treated with BPA. Furthermore a history of cerebrovascular disease is a risk factor for postoperative stroke and mortality22,23. We judged two patients as non-operable owing to their history of cerebrovascular disease. In addition, three patients with a history of cancer were considered to have non-operable CTEPH. Since evidence for the safety of PEA in cancer patients is still lacking, we opted for BPA instead of PEA to treat patients with concomitant cancer. Advanced age should be considered when assessing operability. Previous reports have shown good outcomes with PEA in patients aged up to 86 years20; however, age over 60 years is a known risk factor for in-hospital mortality after PEA24. In our study, there were two in-hospital deaths after PEA in patients who were over 75 years of age. In the BPA group, 80% of patients were over 60 years of age, but the procedures were performed safely. Based on these findings, patients judged as non-operable owing to comorbidity or advanced age can be good candidates for BPA.
Another critical factor in the assessment of operability is clot accessibility. A previous report has shown that approximately half of the cases are judged as non-operable owing to clot inaccessibility5. Generally, patients with surgical classification of disease types I and II have excellent outcomes; however, disease types III and IV are associated with higher perioperative mortality caused by an insufficient improvement of haemodynamics17. The San Diego group has reported that distal segmental and subsegmental lesions are fully accessible, but the outcomes are unsatisfactory17,20. In our study, 24.5% (13/53 patients) of CTEPH patients were judged as non-operable mainly owing to clot inaccessibility. In those patients, BPA effectively accessed distal lesions and improved the haemodynamics. BPA could be promising for surgically inaccessible CTEPH.
Residual PH is one of the adverse events after PEA that is related to in-hospital mortality, with an incidence of 5-35% after PEA25,26. In this study, four patients (16.7%) had residual PH. After hospital discharge, the midterm outcome of residual PH is good27; however, the long-term outcome remains unknown. Furthermore, it is suggested that these patients have “residual CTEPH”, since the recurrence of CTEPH is very rare28, and inadequate surgical clearance of embolic materials in distal branches can be associated with residual PH and worsening of outcomes26,27,29. In the present study, two patients in the BPA group had residual PH after PEA. PAG of both patients demonstrated residual embolic material in the distal PA. In these patients, haemodynamics and symptoms were satisfactorily improved by BPA. Since the risk of reoperation in cardiovascular diseases is high30, BPA can be an alternative therapy in selected patients with residual PH.
Limitations
There are several limitations of this study. The main limitation is the retrospective observational nature of the study design, combined with the fact that the number of patients was small, and the data were collected in a single centre. A multicentre, prospective study is needed for further evaluation to determine the role of BPA. Second, the long-term efficacy of BPA was not evaluated in this study; furthermore, unlike PEA, no study has reported the long-term efficacy of BPA. In addition, restenosis of the PA after BPA was not fully investigated, although in the short term there appeared to be no restenosis after BPA. The influence of small-vessel disease in CTEPH2 on the outcome after BPA is also unknown. Further, long-term analysis will be needed to evaluate the true efficacy of BPA. Finally, the assessment of operability, especially surgical accessibility, remains challenging. Preoperative assessment with modern imaging modalities cannot be easily applied to the evaluation of disease type, and intraoperative assessment is the only way to assess the real type of disease or the accessibility to the dissection plane of the intima. Furthermore, the assessment of operability depends in part on the experience of the institution5. A consensus regarding the assessment of operability is needed in order to determine the role of BPA.
In this regard, BPA is still an experimental therapy and awaits a stronger position in the CTEPH therapy algorithm by the accumulation of clinical evidence. Nevertheless, BPA could provide an additional therapeutic option for CTEPH patients.
Conclusion
In conclusion, this is the first study to evaluate the efficacy and safety of BPA for non-operable CTEPH using as reference the efficacy and safety achieved by PEA for operable CTEPH. Most CTEPH patients can be treated satisfactorily with either BPA or PEA, and this treatment strategy combined with medical therapy, including riociguat, may notably improve prognosis in patients with almost all types of CTEPH.
| Impact on daily practice While pulmonary endarterectory (PEA) is the gold standard for a treatment of chronic thromboembolic pulmonary hypertension (CTEPH) patients, up to 40% of CTEPH patients are judged as non-operable due to distal thromboembolism or comorbidities. Although riociguat has recently been approved for treatment of CTEPH patients, its efficacy is still limited as compared to that of PEA. The present study demonstrated that the efficacy and safety of balloon pulmonary angioplasty (BPA) for non-operable CTEPH patients were similar to those achieved using PEA for operable cases and that, in daily practice, balloon pulmonary angioplasty could provide an alternative therapeutic option for non-operable CTEPH patients. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. A representative pulmonary angiogram of the segmental pulmonary artery (left A10) before angioplasty.
Moving image 2. Image of passing a 0.014-inch guidewire across a lesion.
Moving image 3. Pulmonary angiogram just after dilatation with a 5 mm balloon at 8 atm.
Moving image 4. Pulmonary angiogram four months after the procedures.
Moving image 5. Representative intravascular ultrasound imaging before angioplasty.
Moving image 6. Intravascular ultrasound imaging just after angioplasty.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. A representative pulmonary angiogram of the segmental pulmonary artery (left A10) before angioplasty.
Moving image 2. Image of passing a 0.014-inch guidewire across a lesion.
Moving image 3. Pulmonary angiogram just after dilatation with a 5 mm balloon at 8 atm.
Moving image 4. Pulmonary angiogram four months after the procedures.
Moving image 5. Representative intravascular ultrasound imaging before angioplasty.
Moving image 6. Intravascular ultrasound imaging just after angioplasty.

