
Abstract
Aims: Chronic thromboembolic pulmonary hypertension (CTEPH) is characterised by organised thrombotic obliteration of major vessels and small-vessel arteriopathy in the non-thrombosed vessels. The aim of this study was to investigate the impact of balloon pulmonary angioplasty (BPA) on the non-BPA-side pulmonary vasculature in patients with CTEPH.
Methods and results: This study explored the outcomes of 20 unilateral BPA sessions in 13 CTEPH patients. We measured the pulmonary vascular resistance (PVR), pulmonary artery (PA) flow in the BPA-side and non-BPA-side lungs, respectively, using phase contrast MRI and cardiac catheterisation. The interval from BPA to the follow-up evaluation was 92.8±52.0 days. A single session of BPA decreased mean PA pressure from 37.4±6.2 to 30.9±6.5 mmHg (p<0.001). In the BPA side, BPA increased the PA flow from 1.58±0.65 to 1.95±0.62 L/min (p=0.001) and decreased the PVR from 27.3±27.4 to 14.4±9.0 Wood units (p=0.004). In contrast, it decreased both the non-BPA-side PA flow from 2.25±0.64 to 1.90±0.23 L/min (p=0.008) and the non-BPA-side PVR from 14.8±6.6 to 12.8±3.9 Wood units (p=0.01).
Conclusions: BPA could relieve haemodynamic stress towards the non-BPA-side vasculature and decrease its PVR in patients with CTEPH, suggesting that it can suppress or regress the progression of the small-vessel arteriopathy in non-BPA-side vasculature, presumably due to haemodynamic unloading.
Abbreviations
BPA: balloon pulmonary angioplasty
CTEPH: chronic thromboembolic pulmonary hypertension
PA: pulmonary artery
PC-MRI: phase contrast magnetic resonance imaging
PVR: pulmonary vascular resistance
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterised not only by organised thrombotic obliteration of major vessels but also by small-vessel arteriopathy that are indistinguishable from the findings in pulmonary arterial hypertension1-3. The pathogenesis of small-vessel arteriopathy in CTEPH is unclear but widely considered to be a consequence of excessive blood flow and pressure1. Balloon pulmonary angioplasty (BPA) has emerged as a promising therapy for CTEPH patients who are unsuitable for pulmonary endarterectomy4,5. BPA dilates thrombosed lesions in major vessels and consequently improves the peripheral blood flow and pulmonary vascular resistance (PVR). On the other hand, BPA theoretically relieves the excessive flow and pressure in the non-BPA vasculature including small-vessel arteriopathy (Figure 1). However, the haemodynamic impact of BPA on the non-BPA vasculature has never been investigated. If the excessive haemodynamic stress is the pivotal pathogenesis of small-vessel arteriopathy, it can be expected that BPA suppresses the progression of vascular remodelling in the non-BPA area by reducing haemodynamic stress. On the other hand, if the pathogenesis of small-vessel arteriopathy in CTEPH involves cancer-like proliferation such as pulmonary arterial hypertension6, vascular remodelling in the non-BPA-side lung progresses independently even after BPA. To answer this controversy in the pathogenic mechanisms of small-vessel arteriopathy, serial pathological findings are needed, but repeated lung biopsies are ethically unacceptable in clinical settings. We therefore analysed the PVR, which is the most clinically critical index reflecting the severity of occlusive vessels, instead of the pathological findings. The purpose of this study was to clarify the change in blood flow, pressure and PVR in the non-BPA area before/after BPA by combining phase contrast magnetic resonance imaging (PC-MRI) that can provide local blood flow quantification and invasive cardiac catheterisation.
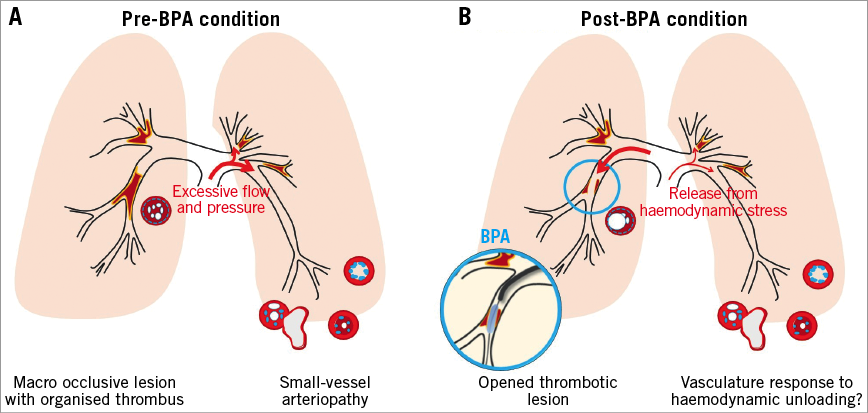
Figure 1. The schematic hypothesis of the study. Chronic thromboembolic pulmonary hypertension has two distinct lesions, 1) macro occlusive lesions with organised thrombus, 2) small-vessel arteriopathy, which is indistinguishable from that of pulmonary arterial hypertension (A). BPA opens macro occlusive lesions and subsequently reduces the pulmonary arterial pressure, correcting the distribution of the pulmonary blood flow. As a result, small-vessel arteriopathy in non-occlusive areas becomes relieved from excessive haemodynamic stress. The aim of this study was to investigate the impact of relief from haemodynamic stress on the non-BPA-side PVR (B). BPA: balloon pulmonary angioplasty
Methods
STUDY DESIGN
The present prospective observational study was approved by the institutional review board (No. 26-190). Twenty BPA sessions were set as the target number for enrolment. The sample size was determined based on feasibility and ethical considerations, since this clinical research was the first exploratory study and there are no preliminary data to calculate a concrete sample size. The timing of the follow-up studies was not restricted and depended on practical circumstances. We started this study in April 2014 and completed enrolment in April 2015. All of the patients provided written informed consent. A patient flow diagram is shown in Figure 2.
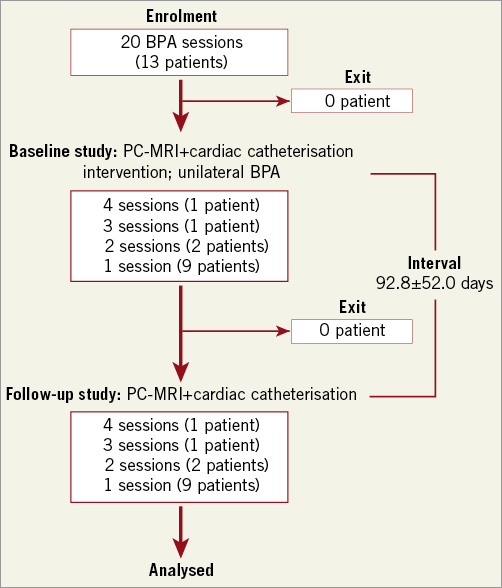
Figure 2. Patient flow diagram. Patients who provided their written informed consent were enrolled. Twenty BPA sessions in overlapping patients (13 patients) were evaluated. The patients underwent PC-MRI for PA flow measurement and cardiac catheterisation for PA pressure measurement before and after BPA. The interval from BPA to follow-up evaluation was 92.8±52.0 days. All of the patients completed the examinations. BPA: balloon pulmonary angioplasty; PA: pulmonary artery; PC-MRI: phase contrast magnetic resonance imaging; PVR: pulmonary vascular resistance
In our hospital, a specialised pulmonary circulation team including cardiologists and surgeons evaluates the operability for pulmonary endarterectomy. We performed computed tomography (CT), lung perfusion single-photon emission computed tomography (SPECT), and pulmonary angiography at the diagnosis and confirmed that all the participants had thrombotic lesions and non-thrombosed open vessels bilaterally. Patients who had proximal occlusions in the non-BPA-side lung were excluded. CTEPH patients who were eligible for this study were further screened using the following inclusion criteria: 1) BPA scheduled in only a unilateral (right or left) pulmonary artery (PA), 2) no vasodilators started or added within two weeks before enrolment, and 3) ≥20 years of age. Patients who required additional vasodilators during the study period were excluded from the analysis. Regarding criterion 1), unilateral BPA was widely accepted. The current BPA statement published by the Japanese Circulation Society and international expert opinion7 recommend performing BPA in a limited area in a staged fashion over multiple procedures in order to avoid substantial reperfusion pulmonary injury4. All participants (n=13) in this study received additional BPA sessions to the opposite-side occlusive lesions after this study following the standard technique8. Consequently, serial BPA (5.4±1.9 sessions/patient) markedly decreased mean PA pressure from 37.4±8.2 to 25.6±4.6 mmHg and PVR from 8.6±3.7 to 3.7±0.8 Wood units without any vasodilators or home oxygen therapy.
MEASUREMENT OF PA FLOW AND PRESSURE
We performed two-dimensional PC-MRI to measure the right, left, and main PA flow prior to BPA. PC-MRI has been widely accepted as a non-invasive measurement of haemodynamic changes in the great arteries9. All patients underwent 3-Tesla MR imaging (Achieva 3.0 T Quasar Dual; Philips Healthcare, Best, the Netherlands) equipped with dual-source parallel radiofrequency transmission, 32-element cardiac phased-array coils used for radiofrequency reception and a four-lead vector cardiogram used for cardiac gating. PC-MRI with a flow-sensitive gradient echo sequence was obtained for 30 cardiac phases. The scan parameters were set as follows: TR=6.5 ms, TE=4.0 ms, flip angle=10 degrees, slice thickness=3 mm, field of view=320 mm×300 mm, acquisition matrix=128×114, and velocity encoded value set to 100-200 cm/s. PA flow (L/min) for the main PA at immediately above the pulmonary valve, and right PA and left PA at the pulmonary hilum were calculated using commercial software (Extended Workspace; Philips Healthcare)10. The scan sections of the main, right and left PAs for PC-MRIs were determined by the consensus of two radiologists. Using the software, firstly the contour of the PA was drawn manually on the initial magnitude image. Next, the contour of the PA was automatically tracked throughout all magnitude images. Then, time-flow curves for the main, right, and left PAs were obtained. If there was an error in the extraction of the PA contour, it was manually corrected.
The PA pressure was measured by cardiac catheterisation at the beginning of BPA. At the follow-up study, we repeated PC-MRI and cardiac catheterisation. We allowed an interval of at least two weeks from the initial BPA session until the evaluation, as a concrete response to BPA takes a few weeks to develop after BPA11,12. After completing the follow-up evaluation, we compared the non-BPA-side PA flow, pressure, and PVR before and after BPA.
CALCULATION OF SELECTIVE (RIGHT AND LEFT) PA FLOW AND PVR
This study required individual right and left PVR measurements. Therefore, we measured the individual right and left PA flow using PC-MRI. The PA pressure was measured by cardiac catheterisation. The selective PVR was then obtained by the following formula based on the Ohm principle:
![]()
where PVR is the pulmonary vascular resistance, PA is the pulmonary artery, and PAW is the pulmonary arterial wedge.
We calculated the selective PVR in combination with the selective PA flow measured by PC-MRI and the PA pressure measured by cardiac catheterisation. PC-MRI and cardiac catheterisation were performed on different days (interval: 3.1±1.9 days). We also recorded the oxygen saturation at rest, since hypoxic vasoconstriction can influence the changes in the non-BPA-side PVR.
STATISTICAL ANALYSIS
Data were expressed as the mean±standard deviation. Comparisons were performed using a paired t-test. A p-value <0.05 was taken to indicate statistical significance. We used the Microsoft Office Excel 2013 software program (Microsoft, Redmond, WA, USA) for all statistical analyses.
Results
PATIENT BASELINE CHARACTERISTICS
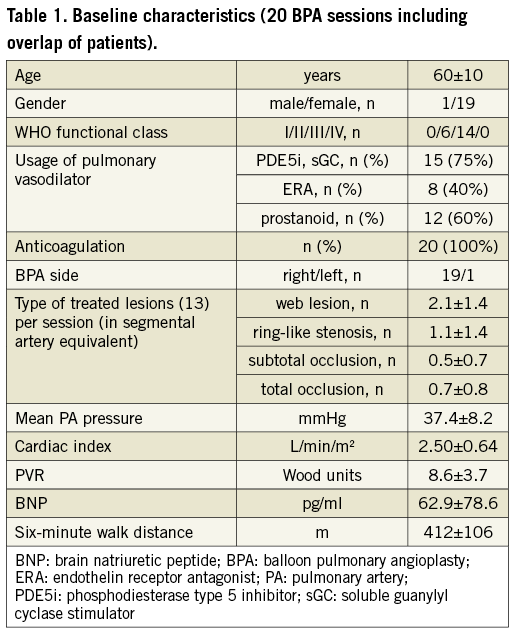
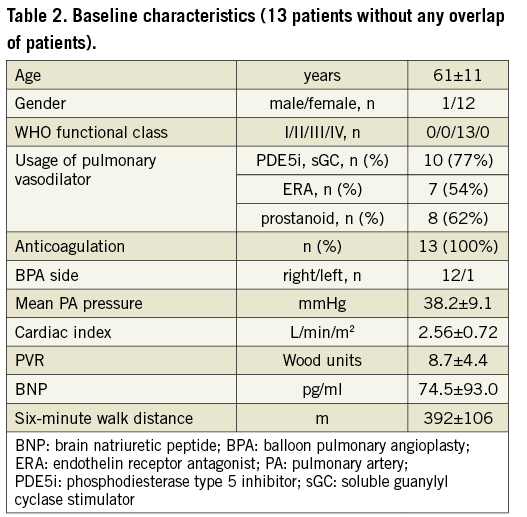
As shown in Figure 2, we evaluated the findings from 20 BPA sessions involving 13 patients. This study allowed the evaluation of different BPA sessions in the same patient. No participants were excluded after enrolment. Table 1 and Table 2 show the baseline patient characteristics. Since no patients had stenotic lesions in the proximal PA, PA pressure in the main PA represents both in bilateral PA pressure. All BPA sessions were performed to unilateral PAs (right: 19 sessions, left: one session) in accordance with the schedule. The number of treated lesions was counted according to the angiographic classification for BPA that was recently proposed13. The predominance of elderly subjects (in their 60s) and female gender reflected the epidemiology of CTEPH in Japan. The PA pressure was 38.2±9.1 mmHg, PVR was 8.7±4.4 Wood units, cardiac index was 2.56±0.72 L/min/m2, brain natriuretic peptide was 74.5±93.0 pg/ml, and six-minute walk distance was 392±106 m, indicating that most participants had mild to moderate disease severity at baseline.
PULMONARY HAEMODYNAMIC CHANGES AFTER BPA
The mean interval from BPA to follow-up was 92.8±52.0 days. Figure 3 summarises the PA pressure, PA flow, and PVR before and after BPA. A single BPA session decreased the mean PA pressure from 37.4±6.2 to 30.9±6.5 mmHg (p<0.001) and PVR from 8.6±3.7 to 6.4±1.9 Wood units (p=0.002). Main PA flow (i.e., cardiac output) did not change after BPA (3.83±1.04 to 3.85±0.69 L/min, p=0.46), probably reflecting the mild disease severity of this study. Focusing on laterality, BPA increased the BPA-side PA flow from 1.58±0.65 to 1.95±0.62 L/min (p=0.001) and decreased the BPA-side PVR from 27.3±27.4 to 14.4±9.0 Wood units (p=0.004). In contrast, BPA decreased both the non-BPA-side PA flow from 2.25±0.64 to 1.90±0.23 L/min (p=0.008) and non-BPA-side PVR from 14.8±6.6 to 12.8±3.9 Wood units (p=0.01). The oxygen saturation level on room air increased from 92.3±4.0% to 93.4±2.7% (p=0.04). In two patients, pulmonary arterial vasodilators were reduced or discontinued by the time of follow-up.
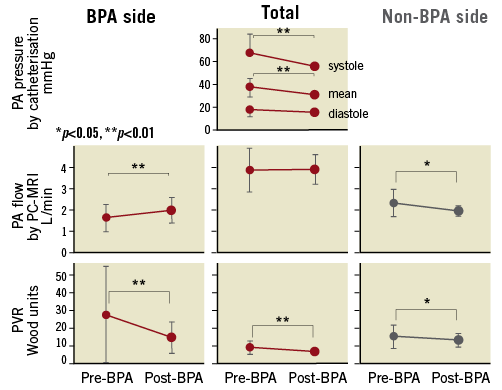
Figure 3. The change in individual haemodynamic parameters after BPA (20 BPA sessions). Changes in pulmonary arterial pressure (upper panel), flow (middle panel), and resistance (bottom) before and after BPA are shown in a total of 20 BPA sessions among 13 patients. BPA-side, non-BPA-side, and total values are distinguished. The main pulmonary artery pressure was the same as the right and left pulmonary arterial pressures.
We performed a sensitivity analysis regarding the initial 13 BPA sessions in the 13 patients to confirm the robustness of the results without any patient overlap. BPA increased the BPA-side PA flow from 1.56±0.72 to 1.99±0.66 L/min (p=0.007) and decreased the BPA-side PVR from 30.2±32.6 to 14.6±10.7 Wood units (p=0.02). In contrast, BPA decreased both the non-BPA-side PA flow from 2.40±0.70 to 1.93±0.24 L/min (p=0.01) and non-BPA-side PVR from 14.4±7.6 to 12.4±4.2 Wood units (p=0.07), in agreement with the tendency of the primary analysis (Table 2, Supplementary Figure 1).
Discussion
This study demonstrated that BPA decreased the BPA-side PVR and total PA pressure, and as a result shifted pulmonary blood flow from the non-BPA-side lung to the BPA-side lung. The spontaneous decrease in the non-BPA-side PVR suggests that haemodynamic unloading suppressed or possibly regressed progression of small-vessel arteriopathy in CTEPH.
IMPACT OF BPA ON THE NON-BPA-SIDE VASCULATURE
BPA decreased the non-BPA-side PVR as well as the BPA-side PVR, despite no intervention being performed. We also found that BPA decreased the non-BPA-side PA flow and PA pressure, suggesting that haemodynamic unloading not only suppresses but also possibly regresses the progression of small-vessel arteriopathy. A sensitivity analysis showed the robustness of the results regardless of the overlap of patients, while it did not reach statistical significance, probably due to the small sample size (Supplementary Figure 1).
Recent experimental studies have demonstrated the important role of haemodynamic stress in the progression of small-vessel arteriopathy14-17. They showed that vessel narrowing and/or occlusion generates turbulent flow, resulting in increased shear stress and evoking inflammation and abnormal cell proliferation. Similarly, it has been reported that high PA pressure induces inflammatory responses in the pulmonary as well as the systemic circulation. On the other hand, we previously reported that reduction of haemodynamic stress (high pressure and excessive flow) by left pulmonary artery banding reversed perivascular inflammation and established pulmonary arteriopathy in the banded lung of the Sugen5416/hypoxia/normoxia-exposed rat18. The mechanism underlying the reverse remodelling remains unclear; however, several clinical studies have demonstrated similar results by haemodynamic unloading19. Levy et al reported a case with pulmonary arterial hypertension which exhibited reverse remodelling in the native lung nine years after single-lung transplantation. They found that single-lung transplantation shifted the PA flow from the native diseased lung into the lung allograft soon after transplantation, which gradually normalised over the years20. Nine years after transplantation, the patient died due to severe pneumonia. An autopsy revealed the resolution of the small-vessel arteriopathy in her native diseased lung. Moser et al reported a 29-case series showing resolution of “vascular steal” after pulmonary endarterectomy - increased blood flow in treated lesions but decreased flow at non-treated lesions after a few weeks21. These findings disappeared 11 months after surgery, suggesting that haemodynamic unloading could restore small-vessel arteriopathy.
We similarly confirmed the “vascular steal” and subsequent blood redistribution on serial scintigraphy in our case which received unilateral BPA (Figure 4). In our case, the “vascular steal” was detected the day after BPA, and blood redistribution was observed by 80 days after BPA. Our case of “vascular steal” suggests that BPA also induces the response of haemodynamic unloading to the PVR even in the midterm (i.e., 80 days after BPA). Therefore, the observation period of the present study (92.8±2.0 days) might be enough to reduce non-BPA-side PVR.
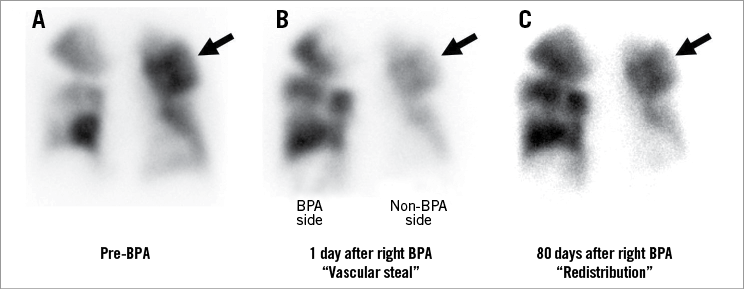
Figure 4. “Vascular steal” and “redistribution” after BPA on lung perfusion scintigraphy. Representative “vascular steal” and “blood redistribution” are shown in a patient with CTEPH who underwent BPA in the right pulmonary arteries. The next day, we observed “vascular steal” on lung perfusion scintigraphy (arrow in panels A and B). However, at follow-up 80 days after BPA, “blood redistribution” was observed (arrow in panel C). The patient’s haemodynamics improved after BPA (mean PA pressure: 59 to 31 mmHg, cardiac index: 3.05 to 4.34 L/min/m2). CTEPH: chronic thromboembolic pulmonary hypertension
In addition, this study first revealed the quantitative response of haemodynamic unloading. The impact of haemodynamic unloading in the present study was limited and not sufficient to improve disease severity. The suppressive or regressive response might be dependent on several factors, such as the time course or severity of underlying arteriopathy. Further studies are required to elucidate what factors are associated with its degree.
POST HOC ANALYSES TO EXAMINE ANY ASSOCIATION WITH CHANGES IN PVR
We conducted a correlation analysis between the changes in the non-BPA-side PVR and baseline haemodynamic parameters. A higher PVR in the non-BPA-side lung at baseline was associated with a positive response in the non-BPA-side PVR (Figure 5), possibly suggesting that the severity of small-vessel remodelling is associated with the favourable response of haemodynamic unloading to the PVR.
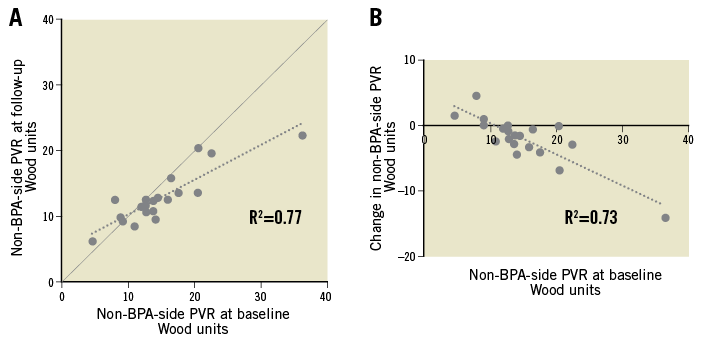
Figure 5. A correlation analysis between baseline and follow-up non-BPA-side pulmonary vascular resistance. The correlations between baseline and follow-up non-BPA-side pulmonary vascular resistance (PVR) are shown (A). The reduction in the non-BPA-side PVR is greater in patients with higher baseline PVR (B).
Study limitations
Several limitations warrant mention in the present study. First, we did not use simultaneous PA flow and pressure to calculate the selective PVR. Muthurangu et al demonstrated the feasibility of simultaneous invasive pressure measurements and MR flow data to quantify the PVR in humans22. However, the availability of the reported technique is limited, since it requires special equipment and a great deal of time. In this study, we confirmed that no participants experienced any clinical worsening during the lag between MRI and cardiac catheterisation. The lag of 3.1±1.9 days can be considered acceptable for the purposes of this study. Second, the accuracy of PC-MRI-guided PA flow measurement might be insufficient. However, several studies have validated its reliability23-26. This study required local flow measurement, as catheter-based flow measurement techniques such as the thermodilution method were not available. PC-MRI is therefore thought to be the best available modality for the purposes of this study. Third, oxygen saturation slightly but significantly improved after BPA. However, the slight improvement (from 92.3±4.0% to 93.4±2.7%) is deemed to have little influence in terms of relief from hypoxic vasoconstriction. Finally, most participants had received long-term pulmonary vasodilators including prostanoids, which might have an antiproliferative effect on pulmonary microcirculation. However, subgroup analysis in the prostanoids and non-prostanoids groups demonstrated that BPA decreased non-BPA-side PVR irrespective of prostanoid usage (Supplementary Figure 2).
Conclusions
This study showed that BPA relieved haemodynamic stress towards the non-BPA-side lung and subsequently decreased the non-BPA-side PVR in patients with CTEPH. Thus, haemodynamic unloading plays a significant role in suppression or possibly regression of small-vessel arteriopathy in these patients. Although further studies to evaluate the pathology in the long term are needed, this finding may improve our understanding of the pathogenic mechanism of small-vessel arteriopathy and help us to develop novel therapeutic strategies for CTEPH.
| Impact on daily practice Small-vessel arteriopathy that develops in patients with CTEPH indistinguishable from that in patients with pulmonary arterial hypertension has been considered to be caused by haemodynamic stress (i.e., excessive blood flow and pressure) towards non-thrombosed patent vessels. The present study demonstrated that: 1) BPA relieves haemodynamic stress towards non-BPA-side vasculature, and 2) non-BPA-side PVR spontaneously reduces in the long term, suggesting that BPA can suppress or regress the development of small-vessel arteriopathy in the non-BPA-side lung. Small-vessel arteriopathy in CTEPH patients might recover by haemodynamic unloading using appropriate BPA. |
Funding
This work was in part supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (26461146) and a grant from Actelion Pharmaceuticals Japan Ltd.
Conflict of interest statement
K. Abe worked in a department endowed by Actelion Pharmaceuticals Japan, and has received a research grant from Mochida Pharmaceutical Co., Ltd. H. Tsutsui has received honoraria from Daiichi Sankyo, Inc., Otsuka Pharmaceutical Co., Ltd, Takeda Pharmaceutical Company Ltd, Mitsubishi Tanabe Pharma Corporation, Boehringer Ingelheim Japan, Inc., Novartis Pharma K.K., Bayer Yakuhin, Ltd, Bristol-Myers Squibb K.K. and Astellas Pharma Inc., and research funding from Actelion Pharmaceuticals Japan, Daiichi Sankyo, Inc., and Astellas Pharma Inc. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. Changes in individual haemodynamic parameters after BPA.
Supplementary Figure 2. Subgroup analysis for prostanoid usage on the change in PVR before and after BPA.
To read the full content of this article, please download the PDF.

