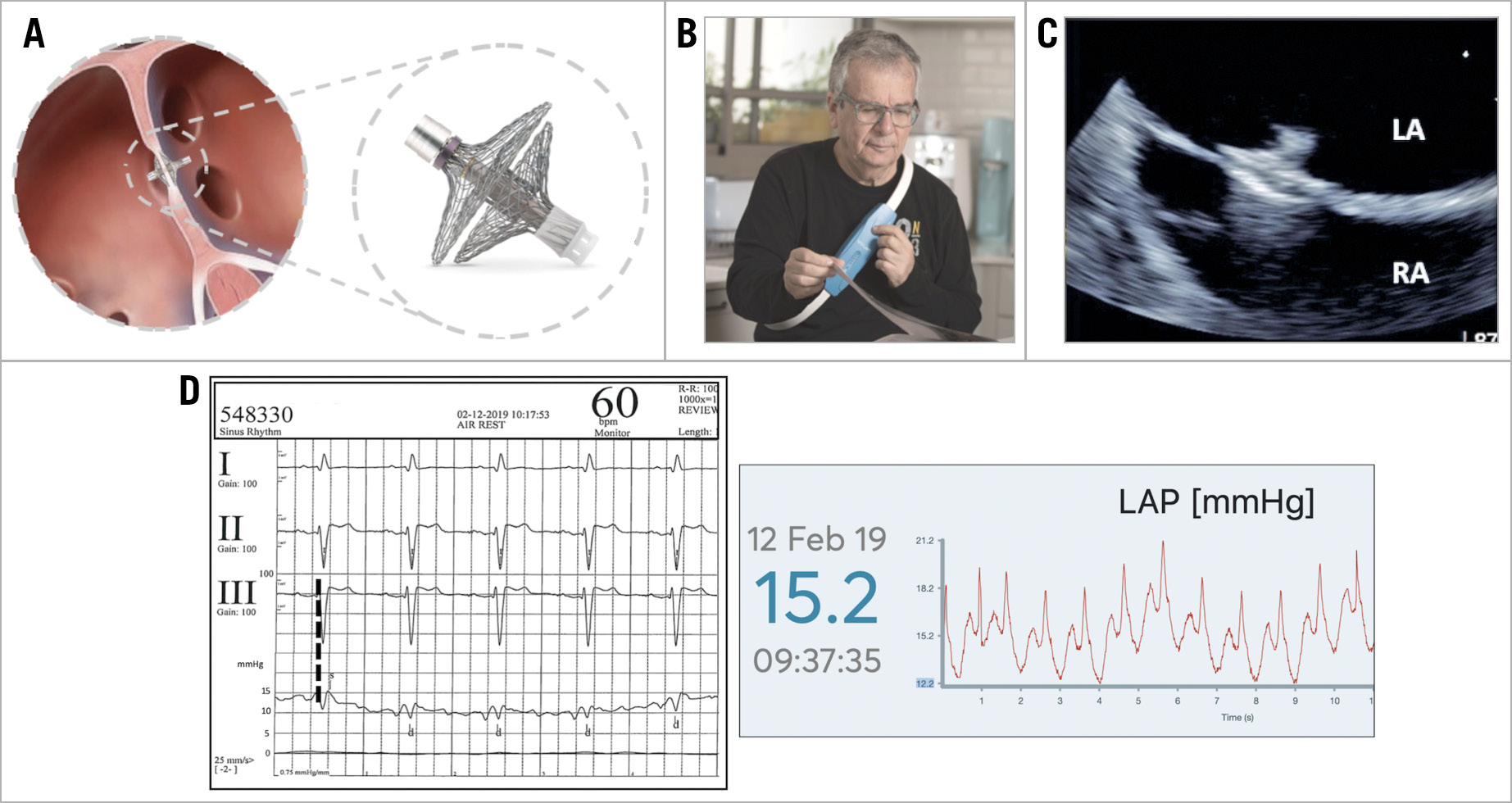
Figure 1. The V-LAP technology. A) The V-LAP implant. B) The external unit. C) TEE image of the implanted V-LAP. D) Simultaneous V-LAP pressure tracings and pulmonary wedge pressure at the moment of the implantation.
Monitoring pressure and adapting therapy to the rapid changes of left ventricular loading conditions is the cornerstone of treatment of acute heart failure in hospitalised patients. This approach has also been translated into the chronic setting, as proved by the results of the only presently commercially available pulmonary pressure monitoring system (CardioMEMS™; Abbott Vascular, Santa Clara, CA, USA) in terms of hospital admissions1and mortality2.
We report the procedural results of a novel permanent sensor recording high-fidelity pressure on the left side of the interatrial septum (V-LAP™; Vectorious Medical Technologies, Tel Aviv, Israel) in two NYHA Class III patients with dilated cardiomyopathy.
The implant (Figure 1A) is composed of a microelectromechanical pressure sensor, positioned on the left atrial side, and an electronic circuit and antenna positioned across the interatrial septum (antenna length 17 mm). These components are held in place by two braided nitinol hemidiscs (16 mm right atrial disc, 18 mm left atrial disc). The anchoring braid is crimped and loaded using a dedicated delivery system deployable through a 12 Fr sheath and remains fully retrievable throughout the delivery process. The implantation procedure requires femoral venous puncture, transoesophageal (TEE) or intracardiac echocardiography guidance, transseptal puncture centrally in the fossa ovalis and final simultaneous measurement of the pulmonary wedge and left atrial pressures. This digital intracardiac pressure sensor enables subsequent bi-directional communication with the external unit (Figure 1B) that also powers the implant and collects data via radiofrequency communication. It is designed to operate with the patient in both the supine and upright positions and during exercise. The implant is designed to be operative and reliable for at least 10 years.
The implantations were performed in December 2018 in Frankfurt and in January 2019 in Florence. The patients treated were male, 72 and 52 years old, with dilated cardiomyopathy (LVEF 32% and 35%, respectively). Both patients were on optimal medical therapy including loop diuretics.
TEE guidance was used in both cases, using deep sedation in one hospital and general anaesthesia in the other (Figure 1C). Standard full heparinisation with an activated clotting time of 250 seconds is required during the procedure. The implantation procedures lasted 7 and 14 minutes, respectively, with high-fidelity signals transmitted to the external unit showing good superimposition of the average pulmonary wedge and left atrial pressure (Figure 1D). Both patients were discharged home the day after the implantation on aspirin and clopidogrel or anticoagulation. No device-related adverse events were registered at follow-up up to 12 months.
Acknowledgements
Dedi Erdheim, Clinical Director of Vectorious Medical Technologies; Elina Soifer, Matan Hershko, Tomer Yahpes and Aviv Lahat, Project & Operation Managers of the same company, gave invaluable help in the technical support.
Conflict of interest statement
C. Di Mario and H. Sievert are members of the Scientific Advisory Board of Vectorious Medical Technologies. C. Di Mario, H. Sievert, K. Sievert and F. Meucci have received institutional grants from Vectorious Medical Technologies.
Supplementary data
To read the full content of this article, please download the PDF.