Abstract
Aims: To demonstrate the feasibility and safety of percutaneous delivery of the novel inflow cannula of the CircuLite® SYNERGY® pocket Micro-pump via transseptal access in the swine model.
Methods and results: After transseptal puncture, the inflow cannula system was advanced into the left atrium (LA) via the right external jugular vein and anchored onto the atrial septum under fluoroscopic and intracardiac echo guidance in 14 acute animals. Subsequently, chronic studies were performed to examine the long-term healing response to the cannula implantation with an artery-LA shunt (n=10) and overall safety of the Micro-pump components (n=6). Acute studies proved the concept of transcatheter delivery of the inflow cannula via superior venous access. The cannula tips were securely anchored in all chronic animals and appropriately endothelialised as early as two weeks. No thrombi or septal damage was observed. For the chronic pump group, device speed of 22,000 rpm (~2.0 L/min) was maintained without any adverse cardiac events. Plasma free haemoglobin assays confirmed the absence of clinically significant haemolysis.
Conclusions: The transcatheter delivery of the inflow cannula via superior venous access to the LA is feasible and safe. This percutaneous delivery presents a significantly less invasive alternative to deliver partial circulatory support devices.
Introduction
Left ventricular assist devices (LVADs) are designed to provide full haemodynamic support1. With flow rates from 5 to 10 L/min that adapt accordingly to pre-load conditions, LVADs achieve haemodynamic profiles leading to beneficial structural and functional myocardial changes1,2. To date, most LVADs are large and their use has been limited to end-stage heart failure due to their high surgical risk, variable device reliability and device-related morbidity3. Smaller devices potentially offer an alternative that could overcome some of these limitations and expand availability.
The SYNERGY® pocket Micro-pump (CircuLite®, Inc., Teaneck, NJ, USA) delivers up to 4.25 L/min of blood flow. Clinical studies have suggested that this type of device improves quality of life and exercise tolerance in New York Heart Association (NYHA) Class IIIb and early Class IV heart failure patients4-6. Currently, this Micro-pump employs a right-sided mini-thoracotomy to insert an inflow cannula in the left atrium (LA) and an outflow graft connected to the right subclavian artery without the use of extracorporeal circulation. Recently, a novel transseptal cannula and delivery system for percutaneous deployment of the inflow cannula to the LA was developed. The purpose of this study was to demonstrate the feasibility and safety of percutaneous transcatheter deployment of this novel inflow cannula via a superior access site to the LA in a porcine model.
Methods
STUDY DESIGN
The study was performed in accordance with the Guide for Care and Use of Laboratory Animals (NIH, 1996) and was approved by the Institutional Animal Care and Use Committee (IACUC). This study comprised both acute and chronic evaluations in a healthy Yorkshire swine model. Swine serve as a suitable model for evaluating transseptal interventions due to the anatomical similarities to the human cardiovascular system7. The initial acute arm of the study (n=14) focused on the delivery of the cannula to the atrial septum using a superior venous access site. The chronic arm of the study evaluated the safety and feasibility of a fully operational device by attaching the hub of the transseptal inflow cannula to an ePTFE outflow graft which connected to the right common carotid artery either through a shunt adapter (n=10) or the CircuLite® SYNERGY® pocket Micro-pump (n=6). The study flow chart is shown in Figure 1.
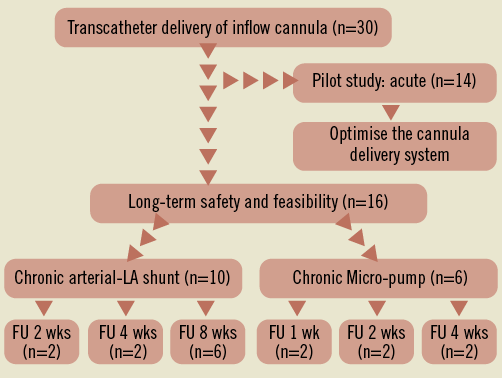
Figure 1. Study design flow chart.
DEVICE DESCRIPTION
The transseptal delivery system consists of a transseptal inflow cannula, obturator, delivery sheath (9 mm OD), and balloon delivery catheter (Figure 2A). The inflow cannula is polyurethane and reinforced with nitinol with a length of 33 cm, an inner diameter of 5 mm and an outer diameter at the distal tip of 6 mm. The distal tip of the inflow cannula is circled by two sets of polymeric structural anchors that are positioned 3 mm apart (Figure 2A, b). The anchors can be deployed from a contracted state to an expanded state and can be fashioned to engage opposite sides of the atrial septum when in the expanded state. Figure 2B shows the inflow cannula loaded into the delivery sheath and the transseptal system prepared for introduction into the right external jugular vein. The percutaneous inflow cannula has been designed to integrate with the CircuLite® SYNERGY® pocket Micro-pump.
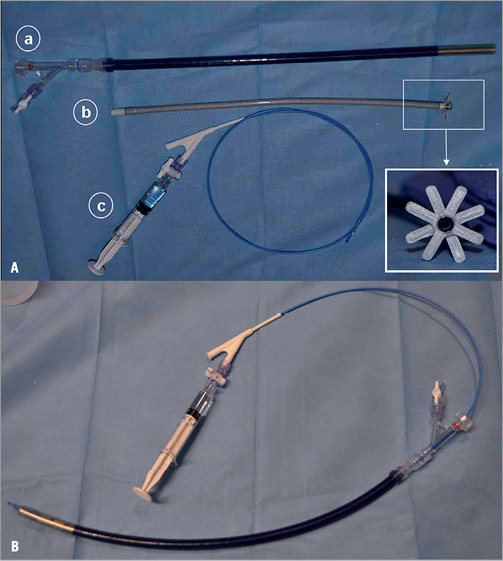
Figure 2. CircuLite® transseptal inflow cannula delivery system. A) The transseptal delivery system consists of a transseptal inflow cannula delivery sheath (9 mm outer diameter)(a), a transseptal cannula (b) and a balloon delivery catheter (c). B) The inflow cannula is loaded into the delivery sheath and the transseptal system is prepared for introduction into the right external jugular vein.
The SYNERGY® pocket Micro-pump has been described previously4-6. In brief, the pump is the size of an AA battery, weighing 25 grams and having an outer body diameter of 14 mm and a length of 53 mm. The pump has a magnetically stabilised, hydrodynamically levitated rotor that spins at 20,000~28,000 rpm. The Micro-pump provides partial circulatory support in chronic heart failure patients and is small enough to be implanted subcutaneously in a “pacemaker-like” pocket through a minimally invasive procedure.
ANIMAL PREPARATION
A total of 30 pigs (weight, 75 to 105 kg) were used. Oral antiplatelet therapy (650 mg aspirin, 150 mg Plavix) was administered one day prior to the procedure in all the animals. The animals were first anaesthetised with Telazol (3 mg/kg IM) then placed on a mechanical ventilator and gas anaesthesia machine while maintained on isoflurane (1 to 3%) supplemented with oxygen. Following appropriate anaesthetic depth, three femoral vascular access sites were obtained in each animal; a 7 Fr sheath (Terumo, Somerset, NJ, USA) was placed in the right femoral artery, and a 10 Fr sheath (Terumo) was placed in the right femoral vein (RFV) and left femoral vein (LFV). Surgical cut-down to access the right external jugular vein (REJV) was performed and a CircuLite® custom venous introducer sheath (10 mm diameter with 12 Fr Check-Flo Performer® [Cook Medical, Bloomington, IN, USA] venous introducer assembly) was inserted. Heparin was administered (100~200 U/kg IV), and bolus injections were repeated as needed (1,000-5,000 U IV) to maintain an activated clotting time (ACT) over 250 seconds.
CANNULA IMPLANTATION PROCEDURE
Intracardiac echocardiography (ICE) guided the transseptal puncture, inflow cannula delivery and deployment. For ICE imaging, a 9 Fr ViewFlex™ 64-element phased-array and bi-directional curve ultrasound catheter (St. Jude Medical, St. Paul, MN, USA) was positioned in the middle of the right atrium (RA) via the LFV. The ICE catheter was rotated clockwise until the standard transseptal view was obtained (Figure 3A). Once ICE identified the fossa ovalis, an 8 Fr dilator from an Agilis NxT steerable sheath (St. Jude Medical) was used to cross the septum from the RFV into the LA. Contrast injected through the dilator confirmed LA access (Figure 3B). A 0.035” HiWire® hydrophilic wire guide (Cook Medical) was then advanced into the LA and used to mark the crossing site after it was positioned in the descending aorta. LA access was obtained via REJV access using an Agilis NxT steerable sheath under ICE guidance (Figure 3C). A second 0.035” HiWire was introduced through the Agilis sheath and advanced into the descending aorta (Figure 3D). Wire exchange was then conducted using a 4 Fr Transit catheter (Cordis Corporation, Miami Lakes, FL, USA) replacing the HiWire with a 0.035” Hi-Torque Supra Core 35 guidewire (Abbott Vascular, Santa Clara, CA, USA). The Supra Core guidewire was advanced into the descending aorta in order to provide maximum support for the delivery of the transseptal inflow cannula system from the REJV.
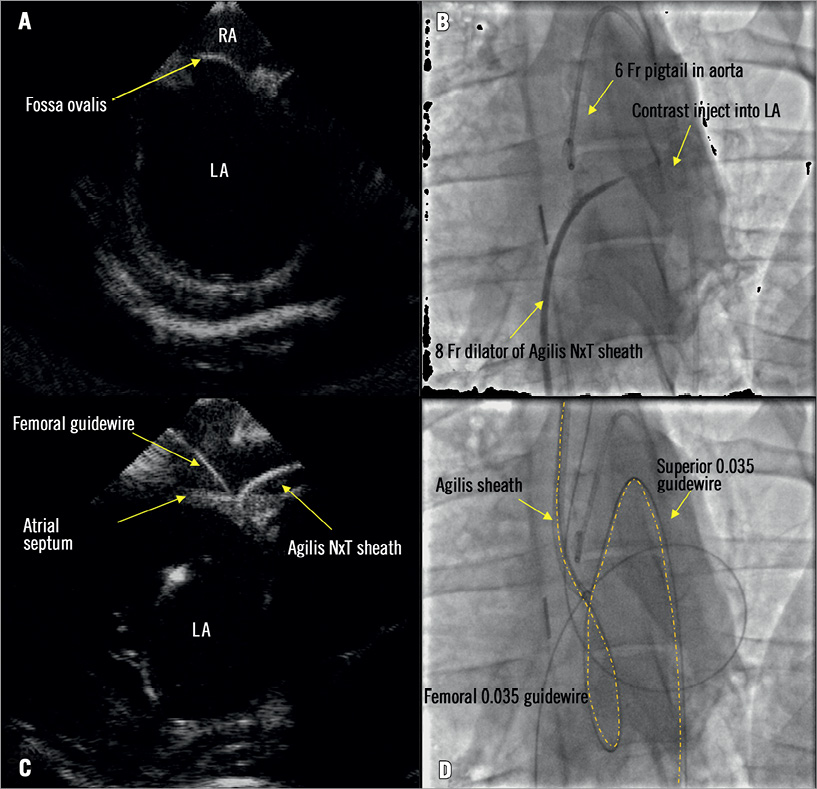
Figure 3. Transseptal access from the right external jugular vein to the left atrium (LA). A) The fossa ovalis was identified by ICE. B) The 8 Fr dilator of the Agilis NxT sheath crossed the atrial septum using transseptal puncture via the femoral artery. C) To re-cross the septum from the superior access, a steerable sheath was inserted into the custom venous introducer and advanced to the right atrium where the tip was deflected towards the septum and crossed into the LA through the femoral puncture site under ICE guidance. D) A guidewire was introduced through the Agilis sheath and advanced to the descending aorta.
The transseptal cannula system was loaded and introduced over the Supra Core guidewire until the tip of the delivery system crossed the septum and was positioned in the LA (Figure 4A). The balloon was deflated and slowly pulled back leaving the tip of the delivery sheath inside the LA (Figure 4B). The delivery catheter balloon was then repositioned and inflated within the inflow cannula tip (Figure 4C), and then slowly pushed forward until the cannula’s left atrial anchors were deployed out of the delivery sheath (Figure 4D) into the LA (Figure 4E). The entire system was then retracted until tenting of the septum was observed, thus ensuring that the LA anchors were in contact with the septal tissue (Figure 4F). With the inflow cannula held in position with the delivery catheter, the delivery sheath was slowly retracted until the right atrial anchors deployed capturing the septal wall (Figure 4G) as confirmed by ICE (Figure 4H). The delivery sheath, Supra Core guidewire, and delivery catheter were removed after the final positions of the LA and RA anchors were confirmed by ICE (Figure 5A). Contrast injected through the cannula lumen verified the position of the inflow cannula body within the RA (Figure 5B).
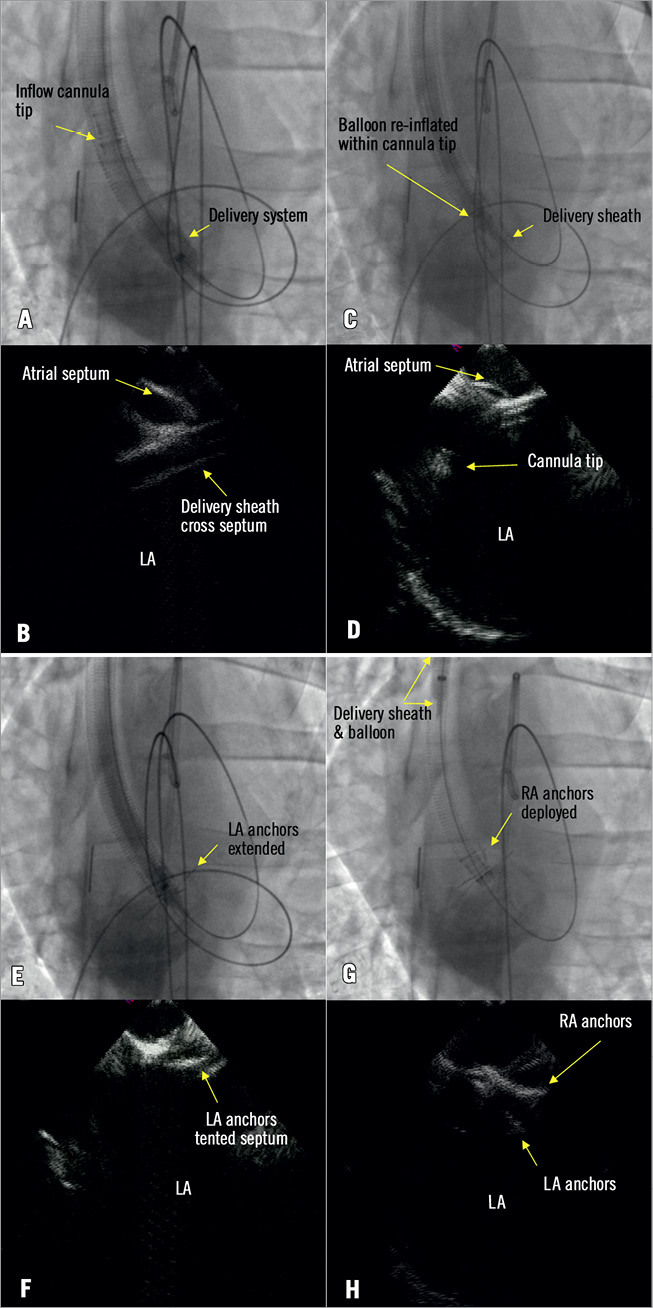
Figure 4. Transseptal inflow cannula delivery. A) The tip of the transseptal inflow cannula delivery system crossed the atrial septum. B) The balloon was deflated and pulled back leaving the tip of the delivery sheath inside the left atrium (LA). C) The balloon was then repositioned and inflated within the inflow cannula tip. D) The cannula tip was pushed out of the delivery sheath. E) LA anchors were extended in the LA. F) The system was pulled back until the LA anchors tented the fossa ovalis. G) & H) The subsequent deployment of the right anchors.
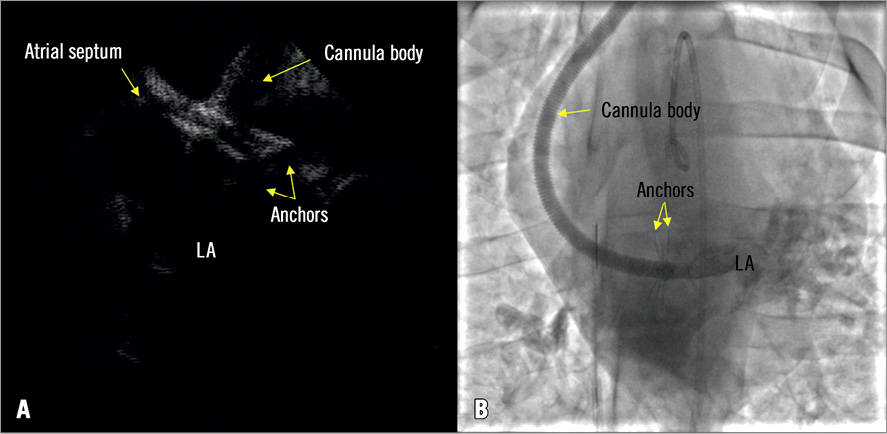
Figure 5. The final positions of the left and right anchors. A) The inflow cannula tip was fixed onto the atrial septum with left and right atrial anchors. B) Contrast was injected through the inflow cannula lumen to verify the position of the inflow cannula body.
CHRONIC ARTERY-LA SHUNT STUDY
After inflow cannula implantation, an artery-LA shunt was used to evaluate the interaction of the anchors with atrial septal tissue and the effect of an implanted cannula in the venous system in the chronic setting (n=10). In lieu of a functioning Micro-pump an adaptor was designed in order to isolate the tissue effects of the cannula without the confounding effects of circulatory assistance. The shunt adaptor is made of titanium and replicates the inflow and outflow components of the SYNERGY® Micro-pump. The outflow graft is made of ePTFE and has an inner diameter of 8 mm. The proximal end of the transseptal inflow cannula was attached to the shunt adaptor, while the outflow graft was attached to the right carotid artery in an end-to-side anastomosis using a 4-0 polypropylene suture (ETHICON, Inc., Johnson & Johnson, Warren, NJ, USA). Shunt patency was confirmed by contrast injection through a 6 Fr Brite Tip® guide catheter (Cordis Corporation, Miami Lakes, FL, USA) with the tip positioned in the right carotid artery via femoral artery access. Animals were anticoagulated with aspirin (81 mg/day) and Plavix (75 mg/day) until termination.
CHRONIC CIRCULITE® SYNERGY® POCKET MICRO-PUMP STUDY
The CircuLite® SYNERGY® pocket Micro-pump was connected to the percutaneously deployed inflow cannula to evaluate the compatibility of the cannula with the pump (n=6). A small pocket in the lower neck was prepared and an end-to-side anastomosis between the Micro-pump outflow graft and the right carotid artery was performed (Figure 6). The Micro-pump cable was tunnelled subcutaneously to the intrascapular region. After completing the initial implant, the Micro-pump was turned on and the speed was increased slowly from 20,000 (~1.8 L/min) to 28,000 rpm (~4.25 L/min) to confirm pump operation and assess atrial collapse. Pump speed was then set to 22,000 rpm (~2.0 L/min) and all incisions were closed using standard methods. All animals were housed individually with a custom jacket to accommodate the external components of the pump. The percutaneous lead from the implanted pump was connected to a control system located outside the pen via an electronic swivel tether (Antron Engineering & Machine, Bellingham, MA, USA) that allowed the animal to move freely. Animals were anticoagulated with daily aspirin (81 mg), Plavix (75 mg) and warfarin (2.5~7.5 mg) until termination. Warfarin dosing was adjusted to achieve an international normalised ratio (INR) between 2.5 to 3.05,6. The pump system remained on for the entire study duration for each animal. Plasma free haemoglobin measurements were taken daily in the first week and then weekly for the duration of pump support.
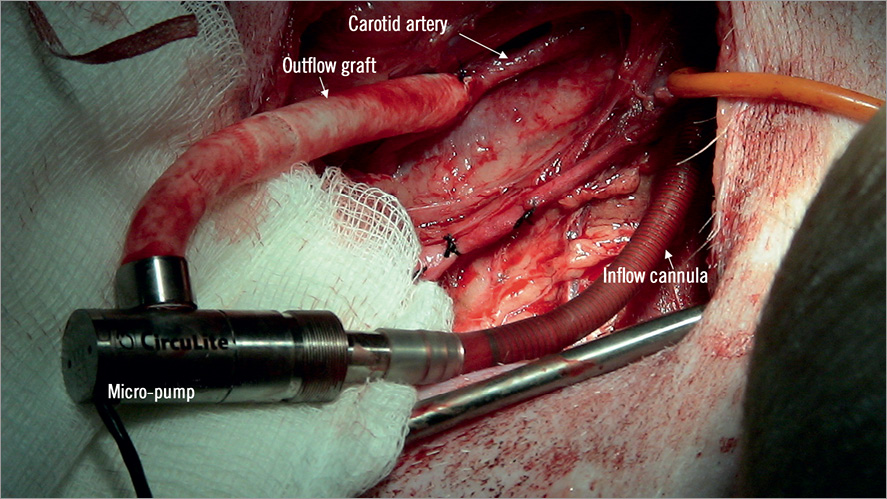
Figure 6. CircuLite® SYNERGY® pocket Micro-pump implantation. A small pocket in the lower right neck region was prepared and an end-to-side anastomosis between the Micro-pump outflow graft and the right carotid artery was performed.
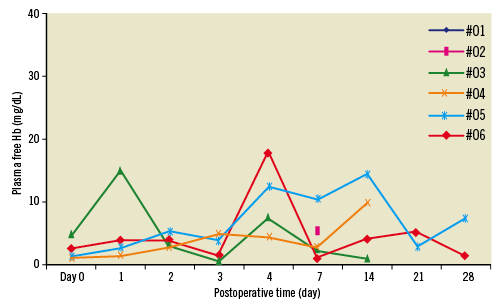
Figure 7. Plasma free haemoglobin values obtained during the four weeks of pump support in six animals.
HISTOLOGICAL EVALUATION
All acute study animals (n=14) were terminated immediately following the inflow cannula implantation procedure. The heart was harvested and both atria dissected. The conditions of the cannula anchors and atrial septum were grossly examined. Chronic artery-LA shunt studies (n=10) were examined at two (n=2), four (n=2) and eight weeks (n=6) following implantation. Chronic on-pump studies (n=6) were followed at one (n=2), two (n=2) and four weeks (n=2). At termination, the Micro-pump was stopped after heparin (10,000 IU) was administered intravenously to minimise any thrombus formation in the system. In all chronic studies (n=16) after the animal was euthanised, the condition of the inflow cannula body, RA and LA anchors and surrounding tissues were examined. The septal implant sites were embedded in Spurr resin, cut, and micropolished at approximately 40-80 microns at three levels and stained with haematoxylin and eosin. In addition, specimens from the lungs, kidneys, liver, and spleen of all animals were grossly and microscopically examined.
DATA ANALYSIS
In the acute arm of the study, superior vein access success, cannula deployment success and ability to re-cross the septum were recorded. All data are expressed as a percentage of total animals enrolled in the acute arm. In the chronic arm of the study, procedure time (expressed as mean±SD) was recorded as well as transseptal puncture success, re-crossing the septum success, post septum dilation events, anchor deployment success, and shunt patency expressed as a percentage of the total animals in this arm of the study. In animals with functioning pumps assessments of plasma free haemoglobin were conducted. The clinically relevant limit of 40 mg/dL was used as a threshold for indicating haemolysis.
Results
INFLOW CANNULA DELIVERY IN ACUTE STUDIES
All acute inflow cannula implants were deployed under fluoroscopic and ICE guidance. Following septal puncture, a guidewire was successfully positioned in the descending aorta in all cases. The ability to re-cross the perforated septum from the REJV was also verified. Following localisation of the perforated septum, a second support guidewire was placed into the distal aorta. Superior vein access was successfully achieved in 12 cases (85.7%). In two cases (14.3%), the inflow cannula could not be delivered due to size restrictions of the REJV (~6 mm). In addition, delivery of the inflow cannula without further dilatation of the septum was achieved in 85.7% of cases. In two animals (14.3%), additional dilatation of the septum using a balloon catheter (10×20 mm) was performed. The acute success rate for inflow cannula deployment onto the atrial septum was 71.4%; necropsy confirmed that the septal crossing was in the region of the foramen ovale. In addition, the inflow cannula anchors were properly deployed in both atria without the presence of acute thrombus formation or any mechanical complications (Figure 8A and Figure 8B).
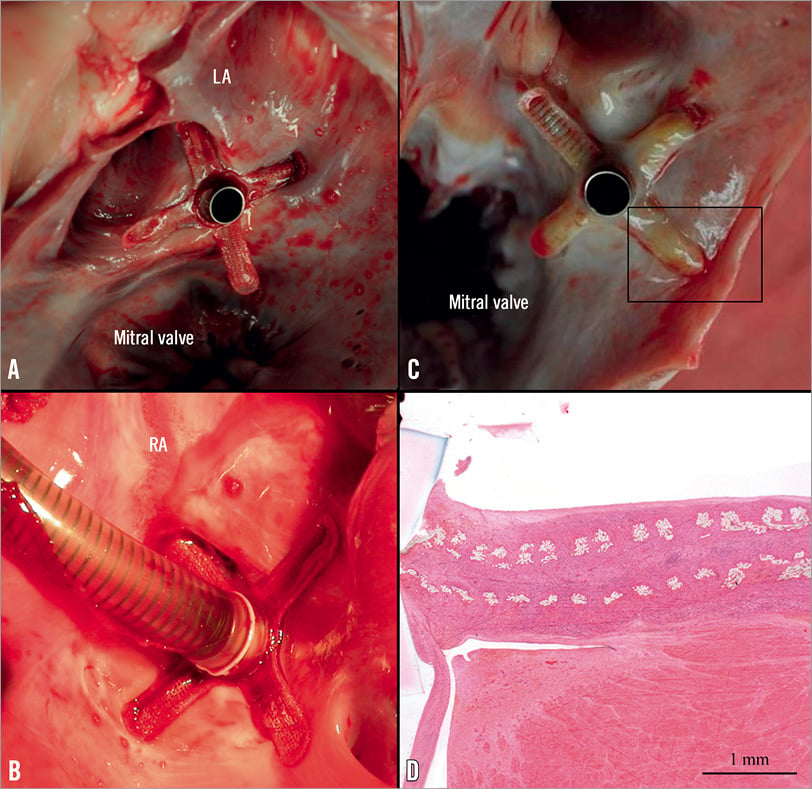
Figure 8. Representative images of accurate deployment of cannula anchors. A) & B) Cannula anchors were nominally deployed in both atria in the acute study. C) Macroscopic evaluation showed complete endothelialisation of the cannula anchors and that the cannula tip was free of tissue formation at 4 weeks. D) Microscopy shows adequate tissue in-growth and expected endothelialisation (image is in perpendicular plane of 7C, haematoxylin and eosin).
INFLOW CANNULA DELIVERY IN CHRONIC STUDIES
The transseptal deployment of the inflow cannula was successful in all chronic animals. The average procedure duration, from transseptal puncture to complete removal of the delivery system, was 53±22 minutes. In addition, the delivery time decreased with operator experience. Under ICE guidance, femoral transseptal puncture was successfully performed at the foramen ovale in all animals. Re-crossing of the septum from the REJV was easily performed in every animal. Additional dilatation of the septum using a peripheral balloon catheter (7×40 mm) was required in three animals (19%).
Anchors were not properly deployed in one instance (6%). This was due to a retraction of the delivery sheath independent of the delivery catheter. In this case, the cannula was recaptured via the delivery sheath and delivery catheter. The cannula was then successfully reloaded into the delivery system and correctly deployed. Inflow cannula anchoring was achieved in all cases as confirmed by ICE imaging. In one case (6%), due to anatomical difficulties, final ICE images showed that one anchor wing remained slightly misplaced relative to the septum wall within the LA. Following inflow cannula delivery, contrast injection through the cannula lumen showed that the contrast remained in the LA without observable shunting into the RA through the septum. There were no significant durable EKG changes procedurally for any animal.
After the inflow cannula implantation, all chronic animals were recovered and survived without any complications. There were no immediate postoperative adverse events for any animals or significant EKG changes. The patency of the artery-LA shunt was confirmed by angiography in all 10 animals at the time of the cannula implantation. An additional six animals underwent implantation of the SYNERGY® Micro-pump and were supported using a pump speed set at 22,000 rpm (~2.0 L/min) from one to four weeks with no adverse cardiac events (100% success rate). Plasma free haemoglobin measurements are summarised in Figure 7, showing the absence of haemolysis. The values generally remained below the clinically relevant limit of 40 mg/dL.
HISTOLOGY EVALUATION OF CHRONIC IMPLANTS
Histological examination showed proper placement of the inflow cannula and anchors in 13 of the 16 (81%) chronic implants (Figure 8C). There were some configuration anomalies (three of the 16 chronic animals, 19%) where one right anchor was misdeployed in the left atrium or within the transseptal channel due to persistent septal defects (<5 mm), leading to interatrial communication and/or projection of the inflow cannula and associated left anchors into the LA (slight malposition). The anchors showed appropriate levels of neointima coverage and organisation. As expected, neointima coverage and organisation were incomplete and immature at one week. From two to eight weeks, the anchors showed appropriate healing, characterised by integration of the anchors into largely stable, organised, endothelialised and mature host tissue with no surface thrombosis in the LA (Figure 8D), except focally in the cases in which the anchoring arms were misplaced and rubbed against the atrial wall. The inflow cannula was encapsulated in the RA. There was no appreciable inflammatory response to the metal components (anchors and tip) or the inflow cannula. The outflow graft showed good integration into a mature and glistening fibrous neointima. In one artery-LA shunt animal (6% of total chronic animals), a cannula fracture was observed at the time of necropsy. This fracture was attributed to a dramatic shift in the shunt adaptor that caused a kink in the curved cannula body that resided in the RA. Gross specimens excised from the lungs, spleen, liver and kidneys were uniformly normal in appearance, with no evidence of congestion, infarction, scarring, thrombus, or embolisation.
Discussion
A number of recent devices have emerged for the support of haemodynamically compromised patients in the interventional setting such as TandemHeart (CardiacAssist, Inc., Pittsburgh, PA, USA), Impella (Abiomed, Inc., Danvers, MA, USA), and other emerging technologies8-10. However, these are percutaneous devices designed for short-term circulatory support. Surgically implanted devices, such as the HeartMate II LVAD (Thoratec Corp., Pleasanton, CA, USA) and HeartWare® Ventricular Assist System (HeartWare, Framingham, MA, USA) have proven to provide significant survival and quality of life benefits especially in INTERMACS 2 and 3 heart failure patients with high risk of short-term mortality. However, adverse events associated with surgery have limited migration of these devices to the large number of patients with end-stage, but not imminently terminal heart failure (e.g., INTERMACS 4 and greater). The percutaneous placement of a partial circulatory support device for long-term use in line with implantable surgical solutions has important clinical potential.
Smaller, less invasive circulatory assist pumps that provide partial haemodynamic support are currently being evaluated in chronic heart failure patients11 . The CircuLite® SYNERGY® pocket Micro-pump is implanted via mini-thoracotomy, and is positioned in a right subclavicular subcutaneous pocket similar to a pacemaker. The inflow cannula tip is inserted into the LA, and the outflow graft connected to the right subclavian artery. It has previously been demonstrated that this partial support pump provides significant and sustained haemodynamic improvements after implantation with increases in arterial pressure and cardiac output accompanied by a reduction in pulmonary capillary wedge pressure4-6. In a recent study6 including 27 patients (ejection fraction 21±6%, pulmonary capillary wedge pressure 28±6 mmHg and cardiac index 2.0±0.4 L/min/m2) in which the SYNERGY® Micro-pump was surgically implanted, patients had significant haemodynamic improvements, with increases in cardiac index to 2.8±0.6 L/min/m2 and reductions in pulmonary capillary wedge pressure (18±7 mmHg) at an average of 9.5±5.5 weeks.
Even though delivery of the SYNERGY® Micro-pump inflow cannula is through a mini-thoracotomy without extracorporeal support, the implantation of this inflow cannula still requires a surgical procedure and has the associated surgical risks which limit use of circulatory support devices. A percutaneously delivered inflow cannula with the SYNERGY® system would further reduce the risks associated with circulatory assist devices and increase utilisation.
In all the animals, transseptal delivery of the inflow cannula was guided by fluoroscopy and ICE. The overall success rate of inflow cannula implantation is 71% in acute and 100% in chronic studies. ICE has emerged as a widespread and useful tool in guiding select cardiac procedures12-14. In the present study, we confirmed that the technique of re-crossing the punctured fossa ovalis from a superior approach under ICE guidance is feasible and may be easily translated into procedures that can be performed in the clinical catheterisation lab setting. In all procedures, the superior stiff guidewire provided sufficient support for advancing the transseptal inflow cannula delivery system. In this series of studies we showed that the transseptal delivery of an inflow cannula is safe and that the anchors on both sides of the atrial septum are effective. There were no cases of inflow cannula thrombosis or infection, and normal healing of the device was observed with microscopic evaluation.
In this swine model, a functioning SYNERGY® Micro-pump or a shunt adaptor was placed in the neck region and the outflow graft was anastomosed to the right carotid artery. This configuration was compulsory due to the anatomical differences that exist between humans and swine, i.e., the subclavian vessels are not accessible in the four-legged animal, as they are in humans. However, the successful superior access from the jugular vein in the swine model provided a platform from which we can translate an equivalent subclavian access to the clinical setting. The long-term patency of the right common carotid artery to the LA shunt via the REJV cannula was confirmed by angiography, gross necropsy and microscopic evaluation. Tissue responses to the fabric, and the low rate of retrograde flow from the common carotid to the LA through the shunt were considered the primary mechanisms that facilitated the tissue overgrowth of the titanium cannula tip in the shunts that did not remain patent. In addition, the animals, supported by the SYNERGY® Micro-pump, tolerated the Micro-pump flow (~2.0 L/min) and survived without any adverse cardiac events or haemolysis, as shown in Figure 7.
The findings from the present study indicate that percutaneous implantation of the transseptal inflow cannula of the CircuLite® SYNERGY® pocket Micro-pump via a superior access is safe and feasible. This implantation presents a much less invasive alternative to current surgical therapies and establishes the tools, techniques, and viability of implanting such a circulatory support device percutaneously. In addition to avoiding the well-known risks of invasive surgery such as bleeding and patient discomfort, patients who are high risk or with other contraindications to surgery have limited therapeutic options; however, the percutaneous delivery of this technology represents a potential novel option for these patients. Paediatric patients and other patients with small body habitus are also suboptimal candidates for assist devices who may benefit from percutaneous technology with small implantable components.
While there is significant clinical potential for this technology, there are certain potential limitations that should be considered. First, there are potential long-term effects of a chronic, large bore indwelling cannula in the subclavian vein and superior vena cava. Even with pacemaker leads, there is a very high rate of subclavian vein thrombosis, which is generally clinically inconsequential. However, with larger bore cannula, it is possible that the superior vena cava could become involved, which could have significant consequences. Although no such problems have been identified in animal studies thus far, this remains as a potential concern upon entering clinical trials with the device described in the present study. Second, with the technique described, the patient must have appropriate peripheral vasculature. Certain heart failure patients with concomitant extensive peripheral vascular diseases involving the subclavian artery are not suitable for this technology. Additionally, the placement of this device may limit future clinical options for certain patients.
The porcine animal model in the heart failure setting is a well-accepted model, but has known limitations. It is possible that in the diseased heart the anatomy of the arterial septum may have changes in compliance. With sufficient operator experience, the transseptal techniques employed in this study should be easily adaptable to the various changes present in the diseased heart. Nevertheless, future research should evaluate this technology in a diseased setting. While we did not observe any lasting EKG changes procedurally and postoperatively, future research should confirm that the safety of this device is comparable to other transseptal implantable devices. In the present study we were able to exchange the cannula when necessary during one procedure and all cannulae were well healed with appropriate endothelialisation and without thrombosis or infection at terminal time points.
The small size of the SYNERGY® pump, combined with a percutaneous delivery, presents a promising new therapeutic option for chronic heart failure patients who are refractory to medical management but who are not sick enough to justify surgical implantation of currently available full support assist devices.
Acknowledgements
The authors would like to acknowledge Alyssa Wagner and Diane Ordanes for their support with animal care.
Funding
This study was sponsored in part by CircuLite, Inc., Teaneck, NJ, USA.
Conflict of interest statement
R. Farnan, D. Burkhoff, and G. Farnan are employees of CircuLite, Inc. S. Kar is a consultant of CircuLite, Inc. The other authors have no conflicts of interest to declare.

