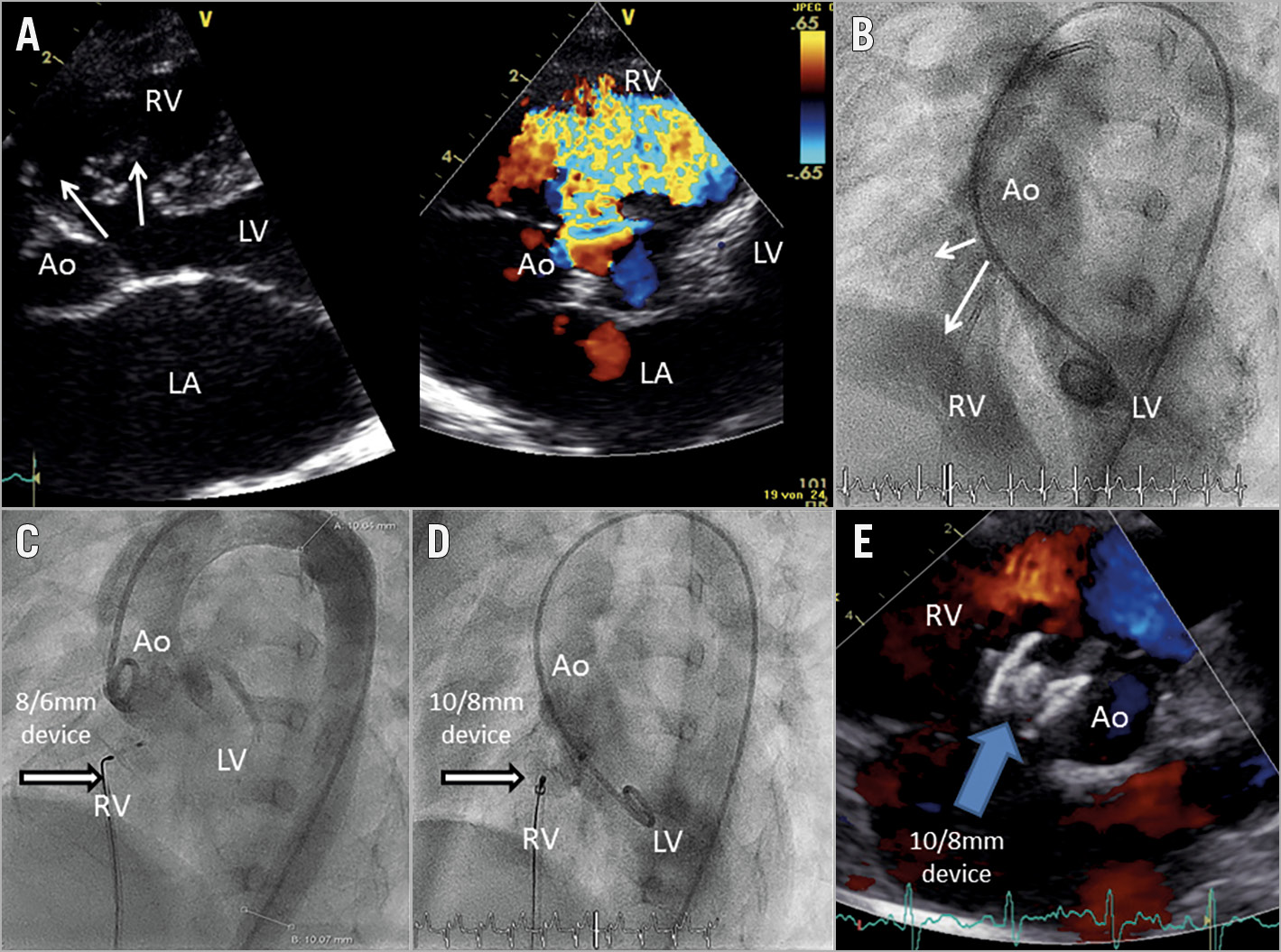
Figure 1. VSD closure. A) Echocardiography before intervention showing a defect with two communications to the RV. B) LV angiography (60° LAO) showing diffuse contrast passage through the defects and clearly recognisable RV. C) Aortography after placement of the 8/6 mm KONAR-MF VSD device with residual shunting through the device and inferior part of the defect. D) Change of the device from 8/6 to a 10/8 mm KONAR-MF VSD device and LV angiography afterwards. E) Echocardiographic result before discharge (day 2) with no residual shunting. Angiographic and echocardiographic results can also be seen in Moving image 1 and Moving image 2.
Percutaneous ventricular septal defect (VSD) closure remains a complex intervention1. In the patient population requiring this intervention, small, flexible and low-profile devices may offer good closure rates with a low rate of complications1,2. Previously, off-label use of patent ductus arteriosus (PDA) devices (e.g., AMPLATZER™ PDA devices [ADO I and II; St. Jude Medical, St. Paul, MN, USA]) has been reported in several case series1,2,3. In May 2018, the KONAR-MF™ multifunctional VSD device (Lifetech, Shenzhen, China) received CE-mark approval for VSD closure after first-in-man implantation in 2013. The KONAR-MF VSD device is made from 144-wire nitinol mesh, is designed as a hybrid between single-disc and double-disc PDA devices, with/without PTFE membrane and can be used through a 4-7 Fr sheath or guiding catheter with a 1.15-1.8-inch inner lumen. It can be screwed at both sides and therefore its placement can be from the left ventricle (LV) or right ventricle (RV), which means retrograde or antegrade.
We describe the first series of European implantations after CE approval in May 2018. Patients with clinical indication for perimembranous VSD closure and who had haemodynamically relevant but restrictive VSDs were selected for an interventional approach. All patients were assessed by transthoracic echocardiography (TTE), electrocardiogram (ECG) and Holter ECG and clinical status between November 2017 and April 2018. All interventions were performed between May and July 2018 under conscious sedation (midazolam and propofol) and local anaesthesia, using a standardised protocol. The aneurysmatic VSDs were passed with a right 4 or 5 Fr modified Amplatz catheter (Cordis Medical, Cashel, Ireland) and an arteriovenous loop was established for passage of the 5-7 Fr implantation sheath (Lifetech). All interventions were guided by transthoracic or transoesophageal echocardiography.
Device implantation was successful in four patients, who had VSDs ranging in size from 2 to 6.5 mm. The following devices were used: 10/8, 9/7, 10/8 and 14/12 mm. In two patients, devices were upsized during the procedure according to significant residual shunting (Figure 1, Moving image 1, Moving image 2). All patients treated underwent standardised follow-up examinations with demonstration of effective function without significant residual shunting or arrhythmia.
This is the first report of successful VSD closure in paediatric and adult patients with the new and CE-marked KONAR-MF VSD device. It has a favourable low profile for antegrade and retrograde implantation; however, more data are needed before possible device-related long-term complications can be ruled out.
Acknowledgements
We thank Anne Wölffel-Gale of the German Heart Center Berlin for editorial assistance and Johannes Nordmeyer, MD, PhD, for preparation of the manuscript.
Conflict of interest statement
S. Schubert is a proctor and consultant for Abbott, Bentley, Edwards Lifesciences, Lifetech and Medtronic. F. Berger is a consultant for Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Angiography after implantation of a 10/Moving image 1.8 mm KONAR-MF VSD device (patient 1).
Moving image 2. Echocardiography after implantation of a 10/8 mm KONAR-MF VSD device on day 2 (patient 1).

