KEYWORDS
Abstract
Aims: Surgical repair used to be the conventional treatment of ruptured sinus of Valsalva aneurysms (RSOVA). Recently many articles have described the percutaneous closure of these defects. We report the successful percutaneous closure of seven cases of RSOVA.
Methods and results: All the procedures were done under local anaesthesia with fluoroscopic and echocardiographic guidance. The defects were closed with nitinol ductal occluders introduced from the venous side after the establishment of an arteriovenous loop. Of the seven patients (four males, mean age 44.8±11 years), six had rupture of the congenital sinus of Valsalva aneurysm, and one had a recurrence following surgical repair. Out of the six patients with aneurysms of the right aortic sinus, four had rupture into the right atrium, one into the right ventricular (RV) inflow and the other into RV outflow. One patient had non-coronary sinus aneurysm rupturing into the right atrium. The size of the distal opening of the aneurysm varied from 2.5 to 12 mm. The left to right shunt flow ratio (Qp/Qs) ranged from 1.5 to 3.6. The size of the nitinol ductal occluders used to close the defects varied from 4 to 16 mm. After a mean follow-up period of 9.3±3 months, all patients remained asymptomatic with no residual flow, aortic valvar insufficiency, or evidence of infection.
Conclusions: RSOVAs can be safely and effectively closed percutaneously using nitinol ductal occluders.
Introduction
Sinus of Valsalva aneurysms are usually congenital. It usually presents in adolescence to early adulthood following rupture. Ruptured sinus of Valsalva aneurysms (RSOVA) are five times more prevalent in Asian countries with a male preponderance of 3:11-3. Although aneurysm formation can occur in any one of the three aortic sinuses, the right and the non-coronary sinuses are the most commonly involved ones1,2.
The haemodynamic effects of RSOVA are profound and nearly 80% of the patients are symptomatic1-3. Surgical repair with patch closure under cardio-pulmonary bypass used to be the conventional treatment of these aneurysms3. The first attempt at percutaneous closure of RSOV was by Cullen et al in 1994 using Rashkind umbrella device4. Since then, few case reports4-8 and case series9-11 have been published. This report describes our experience with seven cases over a period of eighteen months.
Materials and methods
From December 2006 to June 2008, seven patients (four males) were taken up for transcatheter closure of RSOVA. The mean age was 44.8±11 years (range 28 to 62 years). Of the seven cases, six were rupture of congenital sinus of Valsalva aneurysms and the remaining one was a post-surgical recurrence.
All patients were symptomatic in our series. Two of our patients presented with acute dyspnoea and detoriated to NYHA Class IV within a few days. Of the remaining five who presented with dyspnoea on physical exertion, two gradually went into severe congestive heart failure.
The procedure was performed under local anaesthesia with fluoroscopic and transthoracic echocardiographic guidance (Figure 1). Transesophageal echocardiographic guidance was also applied in two of them. All except one had percutaneous access through the right femoral vein and femoral artery. The procedure had to be performed through left femoral arterial access in the remaining patient, since he had a pseudoaneurysm of the right femoral artery. Unfractionated heparin 80 U/Kg and antibiotic prophylaxis was given intravenously to all patients prior to the procedure. Routine right and left cardiac catheterisation was performed to obtain intracardiac pressure data and to assess the magnitude of left to right shunt (Qp / Qs ratio).
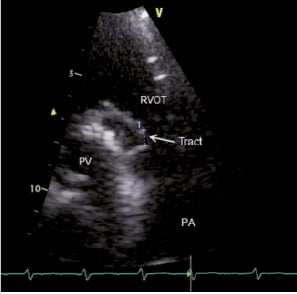
Figure 1. Echocardiographic image of patient number 6 (who had recurrence after surgery) showing the opening of the tract. RVOT: right ventricular outflow tract: PV: prosthetic valve; PA: pulmonary artery; arrow showing the opening into RVOT.
Cine angiographic evaluation of aortic root was performed using pigtail catheter in various orthogonal views. This delineated the opening of RSOVA, the dimensions and its fistulous connections to cardiac chambers. (Figure 2, 4). As coronary anatomy was not sufficiently clear in the aortic root angiography, selective coronary angiography was required in three patients. As RSOVA is usually associated with VSD or aortic valvar insufficiency, left ventriculogram and aortogram were performed in all patients to rule out those possibilities.
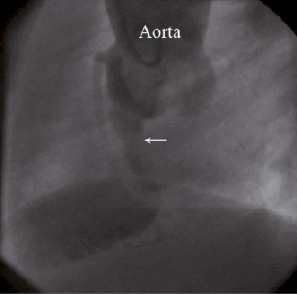
Figure 2. Aortic root angiogram in lateral view (Left Anterior Oblique - LAO 90°) of patient number 3 showing rupture from right SOV to RV, arrow showing the opening of the tract.
Percutaneous closure of RSOVA was done as already reported2,9-11. Once the size of the defect was ascertained, a 6 Fr Judkins right coronary artery diagnostic catheter was advanced to ascending aorta, and the defect crossed using a 0.035 inch exchange length (300 cms) Terumo™ guidewire (Terumo Corp., Tokyo, Japan). An arteriovenous guidewire circuit was then established by snaring the exchange length wire out to the femoral vein using Goose Neck™ snare5 (EV3 Inc., Plymouth, MN, USA). The right coronary catheter was retained across the aortic arch in order to prevent “cut-through” injury during establishment of the arterio-venous loop.
All the defects were closed with nitinol ductal occluders (NDO), which were similar to Amplatzer Ductal Occluder (ADO). The NDOs were manufactured by two Chinese companies:
1) Heart R™ (Lifetech Scientific Company Ltd., Tianan Cyber park, Shenzen, Peoples Republic of China, www.lifetechmed.com ) and
2) Blockaid™ (Shape Memory Alloy Company Ltd., Shanghai, Peoples Republic of China, www.shsma.com).
Blockaid ductal occluder is a morphological replica of ADO. There is no difference in the deployment techniques of ADO and Blockaid NDO. The materials used for manufacturing both devices are nitinol and polyester fabric. Heart R NDO uses ePTFE in place of polyester fabric. The retention rim of this ductal occluder is 2 mm more than ADO for each size available. The delivery sheath recommended for Heart R NDO is one Fr size larger than that recommended for ADO.
The size of the NDO chosen was based on the measurement derived from aortogram. It was 2-4 mm larger than the narrowest diameter of the distal opening of the aneurysm. Depending on the size of the NDO chosen, the appropriate sized Mullins™ sheath was introduced over the arteriovenous guidewire circuit from the right heart into the ascending aorta through the defect.
Under echocardiographic and fluoroscopic guidance the NDO was introduced from the venous side and deployed across the opening of the RSOVA. We repeated aortography and echocardiography 10 minutes after deployment of the device to assess any residual shunt, coronary ostial occlusion or worsening of aortic insufficiency. After confirming satisfactory positioning, the NDO was released. (Figures 3 and 5)
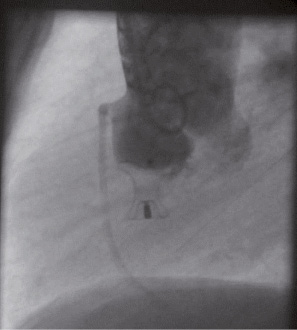
Figure 3. Aortic root angiogram in lateral view (Left Anterior Oblique - LAO 90°) of patient number 3 after successful percutaneous closure of the defect.
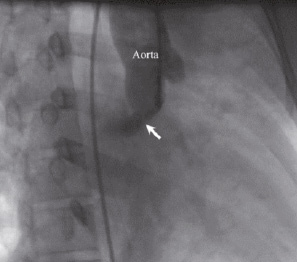
Figure 4. Aortic root angiogram in right anterior oblique (RAO) 30° view of patient number 1 showing the tract (arrow).
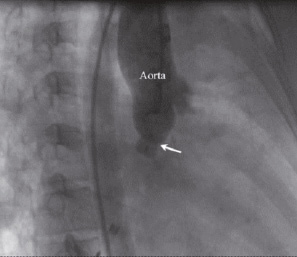
Figure 5. Aortic root angiogram in right anterior oblique (RAO) 30° view of the patient number 1 (the same as seen in Figure 4), after successful percutaneous closure of the defect. (arrow pointing towards the ductal occluder)
Post-procedure, prophylactic antibiotics were routinely administered for five days. Before discharge, all patients underwent clinical examination and evaluation with chest X-ray, ECG and echocardiography.
Results
During the 18 month period from December 2006 to June 2008, seven patients underwent percutaneous closure of RSOVA. The detailed clinical data is shown in Table 1. One patient had non-coronary sinus aneurysm rupture into the right atrium. The remaining six patients had RSOVA of the right sinus. Four of them had rupture into right atrium, one into the right ventricular (RV) inflow and the other into RV outflow.
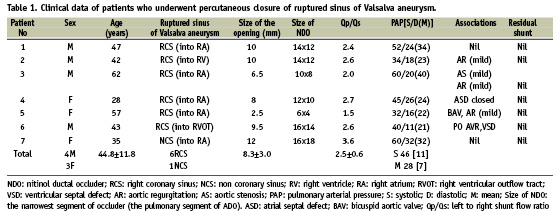
The size of the distal opening measured by aortogram varied from 2.5 to 12 mm. The left to right shunt flow ratio (Qp/Qs) varied from 1.5 to 3.6. All, except one patient, had elevation in pulmonary arterial pressure. Successful occlusion of the RSOVA was achieved in all the seven. The procedure time varied from 56 to 122 minutes (mean 86 minutes) and the fluoroscopy time varied between 14 and 42 minutes (mean 27 minutes). Trivial residual shunt detected in the post-procedure angiogram in two patients disappeared in 24 hours.
One of our cases was a post-surgical recurrence (Figure 1). Eleven years earlier, he had undergone surgical closure of doubly committed ventricular septal defect, aortic valve replacement (28 mm Starr-Edwards prosthesis for severe valvar AR) and patch closure of RSOVA. This time he presented again with a rupture of the right coronary sinus aneurysm to RVOT. This patient was in severe heart failure, multi-organ dysfunction and was a high risk candidate for surgery. Eventually he underwent percutaneous closure successfully.
Another patient, a 35 year old female, presented with acute biventricular failure and was found to have RSOVA of noncoronary sinus to the right atrium. She had congenital right sided diaphragmatic hernia, hypothyroidism and multi-organ dysfunction. She was refused surgery because of the high-risk. She also had a successful percutaneous closure of the defect.
There was no procedure related mortality or other major complications. Two patients had small haematoma’s at the puncture site. Five were discharged after a 2-day observation period in the hospital. Two patients who had heart failure made a slower recovery, and were discharged after 10 days of hospital stay.
Mean follow-up period was 9.3±3 months (8-17 months). There were no device embolisation, infective endocarditis or aortic regurgitation. All patients were put on aspirin 150 mg and clopidogrel 75 mg daily as well as also oral anticoagulants aimed at a target INR of two to three months. After three months, all of them were advised to continue on aspirin alone. The patient having an aortic prosthetic valve was put on warfarin in addition to the aspirin.
Discussion
Sinus of Valsalva aneurysms (SOVAs) occur when there is a congenital defect in the aortic media and incomplete fusion of distal bulbar septum (primitive bulbus cordis) and truncal ridges1,2. There is a relative deficiency of elastic fibres in the affected sinus, which progressively dilates and ruptures over time. SOVAs arise from the right sinus of Valsalva in 80–85% of these cases and from the noncoronary sinus in 5–15%. They rarely arise from the left sinus, as the left coronary cusp embryologically is not derived from bulbar septum. This is distinct from acquired SOVAs, which can occur in any of the sinuses1,2. In this series, out of the seven cases reported, six aneurysms were from the right sinus and one from the non-coronary sinus.
Congenital SOV aneurysms have been associated with other congenital defects –ventricular septal defects, aortic insufficiency, bicuspid aortic valve and persistent left superior vena cava.2 In this report, four patients had associated cardiac anomalies. One of them had an ostium secundum atrial septal defect and another had a ventricular septal defect (VSD) with severe aortic insufficiency. Both these patients had surgical closure of the defects years before their presentation with RSOVA. The remaining two had associated bicuspid aortic valve (BAV). One of them had mild aortic stenosis and regurgitation (AR), while the other only mild AR.
Echocardiographic criteria to diagnose SOVA require the following:
1) the root of the aneurysm must be superior to the aortic annulus
2) saccular appearance of the aneurysm
3) normal size of aortic root2.
All our cases were diagnosed by echocardiography and confirmed at cardiac catheterisation.
It is estimated that rupture of SOVAs occur in 40–76% of patients. Though RSOVAs present typically in Asian populations in the third or fourth decade of life, rupture has been diagnosed across the age spectrum, including infancy in non-Asian populations1,2. We had patients from all age groups starting from the second to seventh decade in our series.
The sudden onset of aorto-cardiac shunting leads to an abrupt increase in venous pressure and a decrease in the aortic diastolic pressure. This precipitates acute symptoms in 37 – 50% of the patients. Patients rapidly develop congestive heart failure due to biventricular volume overload subsequent to systemic-pulmonary shunting2.
RSOVA was previously managed surgically by patching from either side of the defect. With advances in interventional techniques as well as the availability of new tools, it is now possible to tackle these defects percutaneously13. As the defect and rupture occurs above the aortic annulus, the aortic valve leaflets – and often the right coronary ostium – is distant from the opening. These have made percutaneous closure feasible and safe.
Of the few series which reported successful percutaneous closure of RSOVAs (Table 2), the largest was 10 cases by Zhao et al11, followed by eight cases in Arora et al9; ours is the third largest report with seven cases. All our cases had rupture, either from the right or the non-coronary sinus.
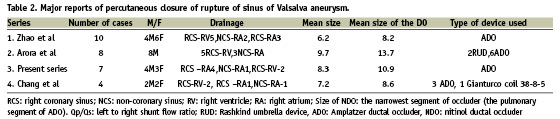
The Rashkind umbrella was the first device used percutaneously to close the RSOVA4; subsequently, the Gianturco coil6 and ADO5,7-11 were used. Cullen et al4 and Rao et al6 used the Rashkind umbrella device and Gianturco coils respectively through the arterial route.
As the NDO can be conveniently delivered from the femoral vein after establishment of arterio-venous loop, the procedure has become effective and safe9-11,13. In all seven cases which we attempted we used nitinol ductal occluders. No reports describing the use of these particular NDOs (Heart R™ or Blockaid™) in closing RSOVAs can be currently found in the literature. Transcatheter closure of two sites of RSOVA in the same patient has also been reported2.
The NDO had to take a very tortuous course in one of our patients who had post-surgical recurrence. This was aided by retaining the arteriovenous loop with the help of an Amplatzer Extra Support™ wire. Arora et al9 has described a patient who underwent percutaneous closure with Amplatzer septal occluder (ASO) which resulted in 40 mmHg gradient across RVOT. Balloon dilatation of RVOT was done with Inoue balloon which brought down the gradient to 10 mmHg. Arora et al9 used balloon sizing of the defects prior to percutaneous closure. We used only echocardiographic (transthoracic and/or trans-oesophageal) and aortographic measurements in all seven of our patients. While Chang et al used trans-oesophageal echocardiography (TEE) in all cases, we used trans-thoracic echocardiography (TTE) guidance in ours. TEE was used additionally for two of our patients. Zhao et al used measurements from aortogram for selection of the devices. Recently 3D echocardiography is applied to aid in the percutaneous closure of RSOVA14.
Percutaneous closure of RSOVA has also been reported along with percutaneous treatment of other cardiac lesions. Rao et al6 has reported balloon dilatation of aortic coarctation and Cui et al16 reported closure of an ASD along with percutaneous closure of RSOVA.
Even though the mortality following surgical closure is low (<2%), potential morbidity from cardiopulmonary bypass and thoracotomy are underlying hazards3,9. Haemodynamically unstable patients with multi-organ dysfunction9,15 are at high-risk for surgical procedures. They may, however, be candidates for percutaneous closure, as the two patients (patients 6 & 7 – Table 1) in our series.
There is only one report about complications following transcatheter closure of RSOV. Arora et al9 has reported an obstruction of the right ventricular outflow tract and persistent haemolysis after transcatheter closure of RSOVA. That patient was finally referred for surgical repair.
All our patients showed good short-term and intermediate term outcomes. At mean follow-up of 9.3 months, all patients were in NYHA Class I with no residual shunt, infection or any symptoms pertaining to the device. Arora et al9, who reported follow-up of 2-96 months, showed six of their seven patients as remaining asymptomatic. Zhao et al11 also reported symptomatic improvement in all 10 of their patients with no complications at three month follow-up. Chang et al10 also reported good outcomes at 3-18 months follow-up.
Conclusion
The use of nitinol ductal occluders has the distinct advantage of being user friendly; they provide easy retrievability as well, along with an ability to reposition, allowing percutaneous closure of RSOV to become an easy, safe and effective procedure.

