Abstract
Aims: To assess fractional flow reserve (FFR) variability in case of arterial hypotension in the clinical setting. FFR measurement is supposed to be independent of haemodynamics; there is, however, a strong relationship between trans-stenotic pressure variation and coronary flow. Non-clinical models suggest an inverse relationship between arterial pressure and FFR, but no clinical data have as yet confirmed this hypothesis.
Methods and results: In case of arterial hypotension (mean arterial pressure [Pa] ≤80 mmHg) during routine clinical FFR measurement (FFR1), a second measurement (FFR2) was performed after pressure normalisation by 0.5 mg IV phenylephrine. Fourteen intermediate chronic stenoses (%DS 58±21%, FFR1= 0.81±11) in 12 male patients showed 70±10 mmHg Pa at the time of measurement. After phenylephrine, Pa increased to 101±14 mmHg and FFR2 decreased to 0.75±12 (p<0.001) without heart rate variation. After Pa elevation, 40% of cases with FFR1 >0.80 changed to FFR2 ≤0.80.
Conclusions: In the present study, in case of arterial hypotension, FFR decreased with rising pressure. Whether repeated FFR measurement after haemodynamic normalisation is of clinical benefit remains at this point speculative and should be validated in a larger data set.
Abbreviations
CFR: coronary flow reserve
FFR: fractional flow reserve
IC: intracoronary
IV: intravenous
LV: left ventricular
Pa: mean arterial pressure
Pzf: zero-flow pressure
Introduction
Fractional flow reserve assesses the functional impact of coronary stenosis, and a threshold of 0.75 corresponds to a significant stenosis1: due to a limited grey zone, FFR ≤0.80 is the recommended threshold for treatment2. Compared to other functional assessment measurements such as coronary flow reserve, FFR, based on pressure measurement under maximal hyperaemia, is more reproducible and moreover independent of haemodynamic variables such as heart rate and arterial pressure3. Compared to angiography, FFR is associated with better prognosis in angioplasty4,5. Its ease of implementation and reliability make it a routine clinical component of diagnostic coronarography, recommended in the absence of prior non-invasive testing6,7.
In case of stenosis, however, there is a quadratic relation between trans-stenotic pressure variation and coronary flow rate8, and modelling revealed an impact of arterial pressure on FFR values9,10, especially for those approximating the clinical cut-off threshold. In view of the immediate therapeutic impact of FFR and the wide inter-subject haemodynamic variation routinely observed in the cathlab, the present study sought to assess variation in FFR measurement in case of arterial hypotension at the time of examination.
Methods
PATIENTS
This prospective study included patients solely referred for invasive assessment of stable or stabilised angina and showing arterial hypotension during FFR measurement, with the following additional inclusion criteria: normal left ventricular (LV) systolic function, with no signs of ventricular hypertrophy on ECG and/or cardiac echo, no history of acute coronary syndrome in the artery studied by FFR, and no symptoms of heart failure. Vasovagal syndrome was an exclusion criterion.
CORONAROGRAPHY AND FFR MEASUREMENT
Coronarography used either a radial or a femoral approach, with 1 mg intracoronary (IC) nitrate in case of coronary atherosclerosis. Coronary lesion angiography was analysed visually and quantitatively (Centricity® CA1000; GE Healthcare, Little Chalfont, Buckinghamshire, UK). FFR was measured routinely when clinically indicated, using a 5 or 6 Fr catheter after intravenous injection of 5,000 IU heparin and 500 mg aspirin. A 0.014” PressureWire™ Certus™ (St. Jude Medical, St. Paul, MN, USA) was carefully calibrated and then passed through the stenosis. Hyperaemia was achieved by IC injection of 100 to 150 µg adenosine ahead of the first FFR measurement (FFR1). Curve equalisation was systematically checked at end-of-procedure on withdrawal of the FFR wire, with exclusion in case of ≥0.02 deviation.
ARTERIAL HYPOTENSION AT FFR MEASUREMENT AND ELEVATED ARTERIAL PRESSURE
Before adenosine injection, patients with normal clinical tolerance and no vasovagal syndrome were included in case of mean Pa ≤80 mmHg at FFR measurement (FFR1). After pressure elevation by intravenous injection of 0.5 mg phenylephrine, an alpha 1-adrenergic vasoconstrictor, a second FFR measurement (FFR2) was made five minutes after arterial pressure stabilised.
FFR variation slope, in 100 mmHg–1, was calculated from the FFR2-FFR1 differential for the differential mean aortic pressure values (Pa2-Pa1) obtained before and after phenylephrine injection.
STATISTICS
Due to the small sample size, statistical assumptions for normality were checked using the Shapiro-Wilk test. All continuous variables (heart rate, arterial pressure and FFR) showed a W value between 0.915 and 0.970, above the threshold of 0.874 set for p=0.05, suggesting normal distribution. Data were then expressed as means or mean ± standard deviation, depending on the type of variable. The paired Wilcoxon signed-rank test for continuous variables and Fisher’s exact test for categorical variables were used. The significance threshold was set at p<0.05.
Results
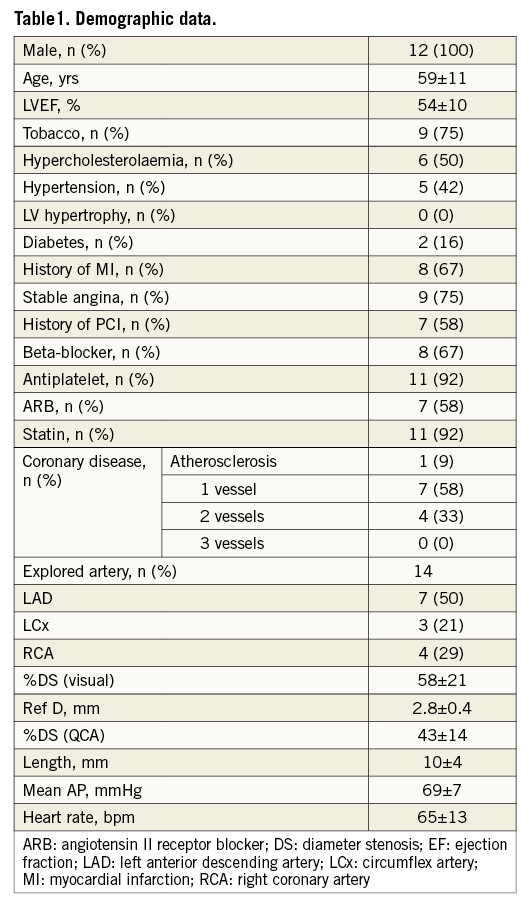
Out of 415 consecutive coronary angiographies using FFR, 12 male patients with 14 intermediate grade coronary stenoses (58±21% diameter stenosis) meeting the inclusion criteria were assessed prospectively (3.3% of the population). The study group’s clinical characteristics were typical of a coronary patient population, and the studied artery was the left anterior descending (LAD) in half of the cases (Table 1).
No clinically significant AV block occurred with adenosine injection. At first FFR measurement (FFR1), mean arterial pressure was 70±10 mmHg (typically, systolic arterial pressure was ≤100 mmHg), with six lesions (43%) showing FFR1 ≤0.80. After pressure elevation to a mean 101±14 mmHg (systolic arterial pressure rose after phenylephrine injection to 140-150 mmHg), all FFR values (FFR2) fell (0.75±12 vs. 0.81±11; p<0.001), with 10 (71%) showing FFR2 ≤0.80 (p<0.05 vs. FFR1; Table 2). The slope of FFR decrease was –20.8±12.5.100.mmHg–1. Interestingly, baseline Pd/Pa, before hyperaemia induction, showed the same relationship on a smaller scale (slope=–9.1±13.0.100.mmHg–1). The four lesions with FFR crossover coincided with the lowest Pa1 (65±7 vs. 73±4 mmHg), and two lesions had FFR1 >0.85 that decreased to <0.75 after phenylephrine. Overall, in case of arterial hypotension, a 31±16 mmHg pressure rise reduced FFR by 0.05±0.03, downgrading 40% of lesions initially considered functionally non-significant (Figure 1). The fall in FFR tended to be greater in angiographically more severe stenoses (–26.6±14.2.100.mmHg–1 for 70-90% stenosis vs. –16.4±9.7.100.mmHg–1 for 40-70% stenosis, p=0.17) and for lower initial arterial pressure (p=0.12; Figure 2). Overall, the observed variability in FFR was 7±4%, and reached 8±3% in case of 70-90% stenosis.
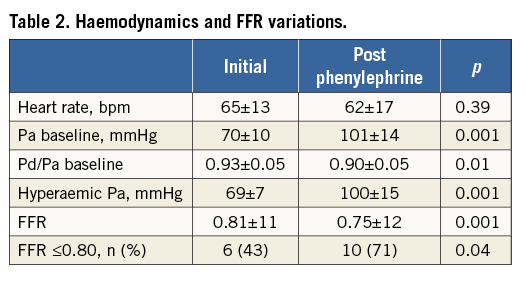
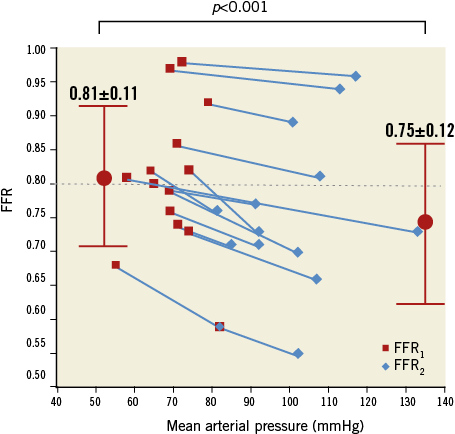
Figure 1. Relationship between FFR (mean±SD) and mean arterial pressure in assessment of a given stenosis.
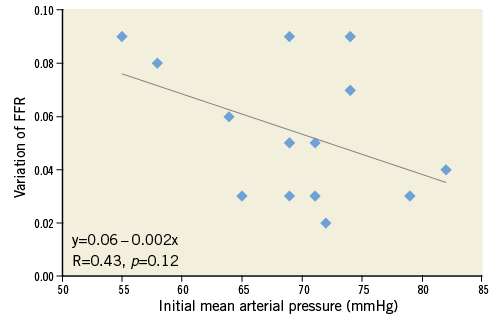
Figure 2. Relationship of FFR variation (expressed as the difference: FFR1-FFR2) according to initial arterial pressure.
Discussion FFR INDEPENDENCE AND HAEMODYNAMICS
FFR is classically considered to be independent of haemodynamic conditions8, with overall inter-subject variation at 4%3, much lower than for coronary flow reserve (approx. 18%). De Bruyne et al demonstrated that variation with change in Pa was only 3.3%3. In case of arterial hypotension (Pa ≤80 mmHg), overall FFR variation is twice as great as usual, especially in the most angiographically severe stenoses (8±3%).
FFR validation studies in humans examined variation under reduction from 100±9 to 79±6 mmHg Pa induced by nitroprusside perfusion3. This vasodilator, however, induced reflex tachycardia (78±10 to 89±12 bpm increase in heart rate: +14%; p<0.05), probably impacting on the Pd-Pv/Pa-Pv ratio9. Experimental studies in dogs also used nitroprusside11, but did not mention heart rate variation. In the present study, Pa variation was obtained without altering heart rate (FC1 65±13 vs. FC2 62±17 bpm; p=NS), using phenylephrine, which has no chronotropic or inotropic effect12. However, α-agonist receptors are present in the coronary circulation tree and could decrease coronary flow in case of coronary stenosis13. Moreover, in case of mild stenosis, intracoronarily injected phentolamine, an α-agonist blocker, has been shown to decrease FFR slightly (from 0.79±0.02 to 0.77±0.02; p=0.03)14. From the point of view of coronary and myocardial physiology, phenylephrine should therefore be able to increase FFR. Despite this, the present results showed that phenylephrine injection decreased FFR significantly, reinforcing the pivotal role of arterial pressure.
It is noteworthy that the present Pa values were lower than those reported with nitroprusside by De Bruyne et al (69±7 mmHg vs. 79±6 mmHg), which may explain why no FFR variation was initially observed3. The paradoxical re-elevation found in FFR values when Pa was reduced by 58±15 mmHg, in a dose-ranging study of adenosine IV, provides further support15.
Patients showing arterial hypotension during FFR measurement are rare (around 3% of the present series) but are nevertheless regularly encountered. The aetiology of this hypotension is not obvious, but certainly involves fasting, isosorbide mononitrate injection and continuation of beta-blockers and/or angiotensin receptor blockers.
MODEL CONFIRMATION
Using a resistive model of epicardial coronary stenosis, Siebes et al analysed the independence of FFR with respect to haemodynamic conditions9 and found overestimation when Pa was reduced or zero-flow pressure (Pzf) increased. FFR was independent of Pa only for Pzf=0; in case of stenosis, it was Pa-dependent, due to the quadratic component of pressure loss according to flow. The present clinical findings are in agreement with these data; in Siebes et al’s simulations9, the FFR variation slope according to Pa could be calculated for a given stenosis: between 50% and 80% stenosis, the slope was about –16.100.mmHg–1, very close to the present value of –20.8±12.5.100.mmHg–1.
Claessens et al modelled the same type of Pa/FFR interaction10 and also found FFR overestimation, by up to 56%, with Pzf elevation. Pzf can be estimated in the clinical setting only by using intracoronary Doppler probe16 but not by FFR. Pzf has, however, frequently been put at 30-40 mmHg16.
CLINICAL IMPLICATIONS
Functional analysis of epicardial coronary stenosis is closely bound to physiological self-regulation phenomena operating at perfusion pressures ranging overall from about 60-70 to 150 mmHg8. If functional assessment is performed at hypotension approximating the lower threshold of self-regulation, then flow change under hyperaemia will be moderate in absolute terms, increasing the risk of relative error. The larger difference between FFR1 and FFR2 noted for the lowest baseline arterial pressure, also not statistically significant, probably reinforces the need for cautious interpretation of FFR in case of arterial hypotension.
To this must be added the intrinsic limits of FFR in terms of Pa, and the uncertainty as to Pzf, exacerbating the risk of overestimation. The present study was based on an arbitrary threshold of 80 mmHg, derived from the generally accepted self-regulatory range, and individual variability could exist. To date, no specific guidelines are available on the optimal arterial pressure range for FFR measurement. In theory, hypotension may lead to underestimation of stenosis severity; however, as yet, no clinical data support this.
In the present study, it was decided to use phenylephrine, to increase blood pressure rapidly; there is no obvious reason why a similar Pa increase should not be achieved by other means (e.g., fluid perfusion), although a validation similar to the present one should in that case be undertaken.
With FFR increasingly used in routine practice, it is important: 1) to detect clinical situations of arterial hypotension; and 2) in case of arterial hypotension, to repeat measurement after optimising the haemodynamic conditions.
Limitations
The sample size was small in this study, even if FFR was observed as falling after Pa elevation in all cases, suggesting a significant physiological relationship between Pa and FFR. Secondly, hyperaemia was obtained by IC adenosine, and extrapolation to IV adenosine measurements is only speculative.
Moreover, measurement was performed during routine practice, and left ventricular filling and venous pressure (Pv) data were not available. Although LV function was normal and no symptom of heart failure was observed, a slight effect of Pv cannot definitely be excluded.
Finally, microcirculatory resistance was not estimated. Two measurement points, however, cannot demonstrate a linear relationship, nor do the models support this. The present slope measurement enables comparison between clinical and model-derived data only along the linear part of the curve.
Conclusions
In this study, in case of arterial hypotension, FFR decreased with rising pressure. This observation confirms modelling data and may raise the issue of the independence of FFR with respect to pressure in a borderline physiological situation; this, however, needs to be confirmed in further studies. Whether repeated FFR measurement after haemodynamic normalisation could show clinical benefit remains at this point speculative and should be validated in a larger data set.
| Impact on daily practice Fractional flow reserve is the standard of care for coronary stenosis assessment, especially when previous non-invasive tests are lacking, and is reported to be independent of haemodynamics. We show, however, that, in the case of borderline physiological arterial pressure (mean arterial pressure ≤80 mmHg), FFR is inversely related to Pa with overestimation of the FFR value in the case of arterial hypotension, leading to underestimation of the explored coronary stenosis. In the case of arterial hypotension, we suggest repeating measurement after optimising the haemodynamic conditions. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

