CASE SUMMARY
BACKGROUND: A 72-year-old man with an infected permanent dual chamber pacemaker.
INVESTIGATION: The patient underwent successful generator removal, although the pacemaker leads were left due to severe entrapment despite energetic external traction. Percutaneous lead extraction was performed and was complicated by tricuspid valve avulsion leading to severe tricuspid regurgitation.
DIAGNOSIS: Entrapment of infected pacemaker lead.
MANAGEMENT: First, a loop around the leads was created using the combination of a gooseneck snare and a wire. Second, a single goose snare was used to remove the remaining severely entrapped piece of lead.
KEYWORDS: pacemaker infection, percutaneous lead extraction, tricuspid regurgitation
PRESENTATION OF THE CASE
A 72-year-old man was admitted to a regional hospital for removal of an infected permanent dual chamber pacemaker. He had undergone a dual chamber pacemaker implantation due to a complete heart block three years before. Physical examination on admission revealed sepsis with haemodynamic impairment secondary to pacemaker infection, remaining febrile. Pseudomonas aeruginosa grew on the cultures of the pacemaker generator’s pocket. Long-lasting antibiotics were started and the external removal of the device was scheduled. The generator removal was performed although direct manual traction of the leads was unsuccessful due to lead entrapment. The procedure was stopped at this point, and the patient was then referred to our institution for surgical removal of the leads. The cardiothoracic surgery team, after another failed attempt at external removal, deemed the risk of open-heart removal as excessive due to clinical instability and frailty. A new epicardial VVI pacemaker was implanted as the heart rhythm was pacemaker-dependent, and percutaneous lead extraction in the catheterisation lab was indicated (Figure1A, Figure 1B, Moving image 1A, Moving image 1B).
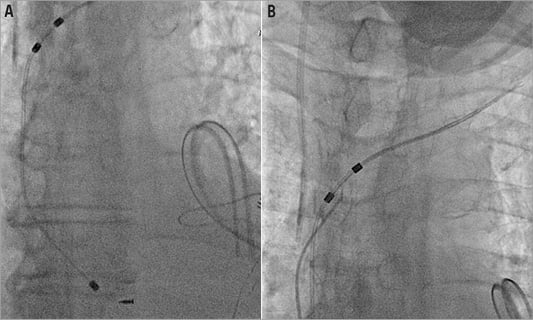
Figure 1. Baseline fluoroscopic images of the broken entrapped lead.
How would I treat?
THE INVITED EXPERTS’ OPINION
The case presented by Asmarats et al is a typical example of a septic endocarditis which requires complete device and lead removal based on current guidelines1. In similar cases we always recommend a stepwise approach. First, intravenous administration of ceftazidime 6 g daily should be initiated for a sensitive pseudomonas aeruginosa. In our centre, urgent full system removal is always performed using general anaesthesia with a back-up surgical team present. A pre-procedural fluoroscopy and venogram would allow us to evaluate lead location, vascular access and the presence of adhesions. Prior to the procedure, an additional sheath would be placed in the contralateral jugular vein and one or both femoral veins to obtain an alternative venous access other than the implant vein, and to position a temporary pacemaker lead into the right ventricular apex if applicable. This access could also be used later if femoral approach is necessary. The first step is usually simple traction using standard stylets in combination with graded traction and/or rotational force. A second attempt is mostly performed by introducing a Liberator® locking stylet (Cook Vascular Inc., Vandergrift, PA, USA) into the conductor lumen to prevent lead disintegration. While introducing the stylet, fluoroscopy reveals quite accurately the location of possible focal adhesion. If there is no accessible lumen in the lead, we use a Bulldog™ device (Cook Vascular Inc.) for external fixation of the pacemaker lead or its internal cables, which also allows direct traction without lead disintegration, as well as extension of the lead so that it can be pulled into a sheath2. In case of proximal adhesion to the subclavian and/or superior caval vein, Byrd dilator sheaths, Evolution® Shortie or Evolution® mechanical dilator sheaths (Cook Vascular Inc.) are used to separate any fibrous or calcified binding adhesions from the leads without any electrical energy delivery. The decision about the use of the above-mentioned devices is based on the location and the length of the adhesion. Advancing a sheath over the lead is performed by using a counter-pressure and counter-traction technique2. After failed superior attempts or in the case of free floating parts due to lead disruption, a Needle’s Eye Snare® (Cook Vascular Inc.) is introduced via a non-implant vein, usually the femoral vein2. We perform this technique exclusively in cases where the tip of the electrode can be freed from the myocardium and when severe proximal adhesions are identified. Usually a successful extraction can be obtained with this latter technique. At the end of every extraction procedure, echocardiography should be performed to exclude pericardial effusion. Using the approach described above, performing more than 60 extractions annually, there has been a need for surgical conversion in only one case in the last five years in our centre. After successful extraction of the device, lead tip and removed tissues are sent to obtain additional microbiology culture. Since gram-negative bacteraemia is less common as a cause, attempts should always be made to determine its source.
In conclusion, we strongly believe that percutaneous lead extractions are high-risk procedures that require careful preparation in any setting. In patients in whom devices were implanted a long time before, extraction procedures should be performed in very experienced centres with a back-up surgical team present. Indeed, a facility with hybrid operating theatre is desirable. A structural stepwise approach is recommended using a wide range of tools and techniques to obtain the ultimate goal: a safe and successful lead extraction. Special attention should be given to avoid lead disintegration and repeated procedures, since these factors significantly increase morbidity and mortality.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
More pacemakers and other cardiac implanted devices mean more infected systems requiring management. Complete removal of the device, leads and other material is necessary to eradicate a systemic infection, but this remains a technically challenging procedure with significant associated mortality and morbidity. It should only be undertaken by experienced clinicians in centres regularly performing the procedure3. Excellent fluoroscopic imaging, the ability to provide immediate cardiopulmonary bypass and a range of extraction tools are mandatory1. Apart from the tip, the lead may be firmly attached to fibrous tissue anywhere along its intravascular course. A carefully planned, sequential approach is necessary to achieve optimal outcomes; each step should not make the next one harder.
Given the lead dwell time, we would not have attempted removal by simple traction alone unless the leads were clearly free within a tract. Pulling on a lead without a locking stylet in place may result in complete lead disruption. As has happened here, once the lead retracts into the vessel, further attempts at extraction are more difficult. Damaging leads with manual traction prevents the use of locking stylets and counter-traction sheath systems to undertake extraction via the implant vein. We usually prepare all leads with locking stylets before starting extraction to avoid loss of inner lumen integrity on both the targeted and non-targeted leads. A mechanical sheath (Evolution; Cook Vascular Inc.) is then used to free fibrous adhesions, with an outer sheath providing controlled counter-traction.
For this case, with an entrapped lead retained endovascularly, we would use a transfemoral approach with a Needle’s Eye Snare® (Cook Vascular Inc.) to grasp a free portion of the lead4. Once captured, gentle traction will usually enable the proximal end of the lead to be withdrawn free from the binding endovascular casts within the subclavian system. Once this portion has been freed, counter-traction can then be used to free the lead tip using standard techniques. If the angulation and approach to the lead tip site are unfavourable, curved sheaths can be used to provide optimal counter-traction, thereby minimising the potential for cardiac injury. If this approach is unsuccessful, a combined femoral and right internal jugular approach, as described by Bongiorni, would be tried next5. This improves the angle of approach to the lead tip, but is more complex and may limit subsequent right-sided venous access. Given the sepsis, every attempt should be made to remove any retained foreign material from within the vascular structures and pocket.
Rather than implanting another permanent system we would usually tunnel an endocardial active fixation pacing lead via the left jugular vein, as a temporary option. This preserves the right side for a future permanent transvenous system once the infection has been treated, and avoids the risk that another permanent pacemaker lead might also become infected. Implanting a permanent endocardial lead in a temporary fashion is less invasive than thoracotomy for placement of an epicardial system.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
The patient underwent transfemoral vein percutaneous catheterisation with a 10 Fr sheath. Medium and large Amplatz GooseNeck™ snares (ev3 Inc., Plymouth, MN, USA) were initially ineffective as was a Dormia basket. The combined use of a GooseNeck snare and a 0.035 inch Teflon coated Cordis® wire (Cordis, Johnson & Johnson, Warren, NJ, USA), placed in parallel and around the leads to create a loop at the level of the superior caval vein and left subclavian vein, was finally successful in catching and removing the proximal end of the lead (Figure 2A-Figure 2C, Moving image 2). However, there was a piece of lead remaining severely entrapped at the tricuspid valve and right ventricle, which could finally be removed by manipulating one 35 mm Amplatz GooseNeck snare (ev3 Inc.) with a Judkins Right 4 6 Fr guiding catheter (Cordis) (Figure 3, Moving image 3, Moving image 4), with a large piece of biological material attached to it (Figure 4A, Figure 4B).
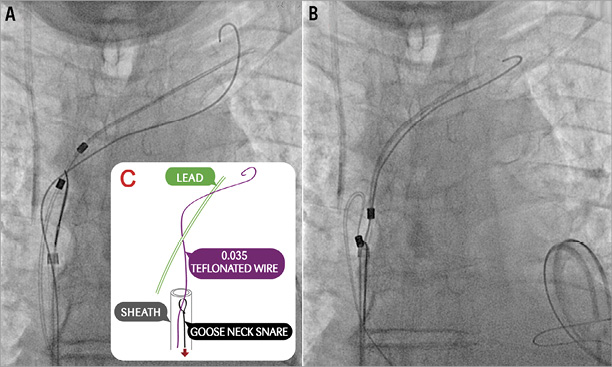
Figure 2. Removal of pacemaker lead. A) & B) Removal of the proximal end of the lead by combination of a GooseNeck snare and a 0.035 inch Teflon coated Cordis® wire placed in parallel and around the leads to create a loop at the level of the superior caval vein and left subclavian vein. C) Diagram showing that the Teflon coated wire was advanced through the snare and inside the same sheath.
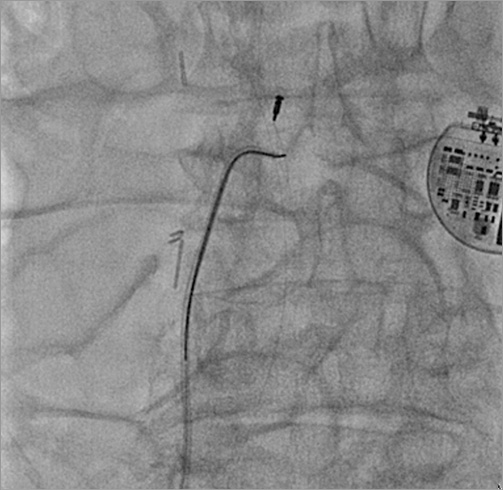
Figure 3. Extraction of the attached remaining piece by means of a 35 mm Amplatz GooseNeck snare (ev3 Inc.) with a Judkins Right 4 6 Fr guiding catheter. On the right side is the epicardial pacemaker.
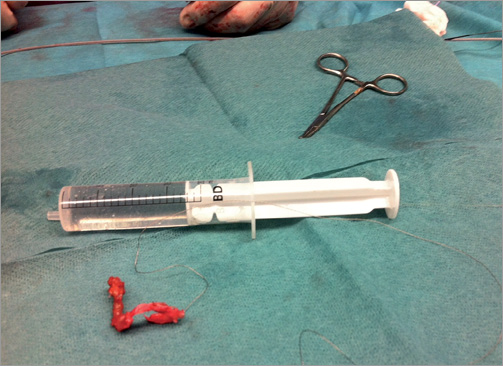
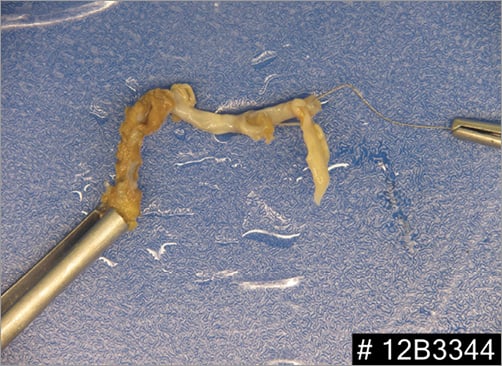
Figure 4. A pacing lead with tricuspid valve avulsed material tightly adhered to it after lead extraction. A) The large endothelialised avulsed tissue placed next to a 20 cc syringe for size comparison. B) Gross photograph of the removed piece.
The post-procedural emergent transthoracic echocardiography (TTE) showed mild pericardial effusion with preserved biventricular function. The patient remained eleven days in the intensive care unit, where he presented septic shock and acute renal failure requiring continuous venovenous haemodiafiltration. Blood cultures were negative, and Pseudomonas aeruginosa grew on the pacemaker lead culture. He recovered well and was transferred to the cardiology ward.
Two weeks after the lead retrieval, the patient developed right-sided cardiac failure symptoms. A planned TTE revealed severe tricuspid regurgitation, which was absent in the previous study before the final pacemaker removal (Figure 5). Histopathology of the biological material attached to the endothelialised lead showed two layers of endothelium and a fibrous layer in the middle of both, which corresponded to tricuspid valve tissue (Figure 6).
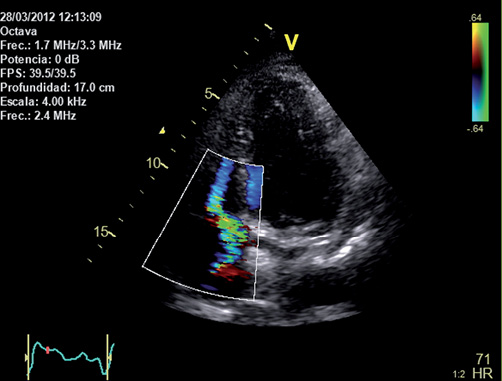
Figure 5. Transthoracic echocardiography study showing a severe tricuspid regurgitation (apical four-chamber view).
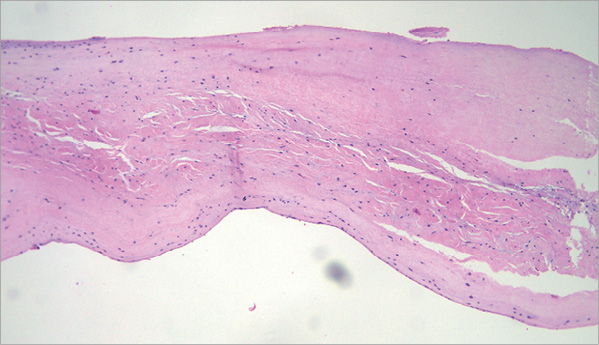
Figure 6. Histopathology of the biological material attached to the endothelialised lead showing two layers of endothelium and a fibrous layer in the middle of both which corresponds to tricuspid valve tissue.
After proper response to diuretics the patient was discharged with medical therapy only. At six-month follow-up, he is in NYHA Class II. Future assessment of clinical and valvular status is warranted to evaluate the need for tricuspid valve repair surgery.
Discussion
Untreated pacemaker-related infection is associated with mortality rates ranging from 31 to 66%, a rate two to three-fold higher than the mortality seen in patients treated with antimicrobial therapy combined with device removal6.
Direct manual traction is probably the simplest method for pacemaker lead extraction. However, it is often unsuccessful for chronically implanted leads and may be related to serious complications. Over the years, new grasping tools such as telescoping sheaths and excimer laser sheaths have been developed to increase the efficacy and reduce the complications of the external approach, especially useful when other techniques fail7. Conversely, a percutaneous extraction procedure or open surgery is needed when a piece of ruptured electrode remains inside the vascular system.
Either manual traction or percutaneous transvenous lead removal may lead to severe complications such as tricuspid valve injury, subclavian vein or superior vena cava tears, right atrium or right ventricle perforation, pneumothorax, haemopericardium, pulmonary embolism, and fatal arrhythmias.
Traumatic tricuspid regurgitation after percutaneous ventricular lead removal is an uncommon but potentially serious complication, sometimes requiring open surgical repair. Most of the tricuspid regurgitation cases related to pacemakers and implantable defibrillator leads occur during implantation since the lead crosses the tricuspid valve and may impair its closure8. Fibrosis and adherences between lead and tricuspid valve develop over time9-12. Many cases of traumatic lesions of the tricuspid apparatus after manual traction or surgical lead extraction have been described13-18. However, severe tricuspid regurgitation following lead removal has rarely been reported in the modern era of percutaneous transvenous lead extraction19-21. A study evaluating the effect of lead extraction on tricuspid valve regurgitation using transoesophageal echocardiography showed a 12% incidence of new tricuspid regurgitation, with a trend to increasing damage in patients where laser techniques had been performed22. Likewise, a prospective five-year single study, during which 237 leads were removed from 208 patients, found tricuspid regurgitation in 19 patients (9.1%), 14 of which were severe cases. Nine of these 14 cases developed new right-sided heart failure. Three independent risk factors were identified: use of laser sheath, combination of laser sheath and lasso, and female sex19.
We conclude that the technique of a wire around an entrapped pacemaker lead before advancing a goose snare allows it to catch the lead more easily and faster than with a lone goose snare.
After a laborious percutaneous retrieval of an infected pacemaker lead, careful clinical and echocardiographic follow-up should always be indicated to identify and treat complications promptly.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Baseline fluoroscopic images of the broken entrapped lead.
Moving image 2. Removal of the proximal end of the lead by combination of a GooseNeck snare and a 0.035 inch Teflon coated Cordis® wire placed in parallel and around the leads to create a loop at the level of the superior caval vein and left subclavian vein.
Moving image 3 and Moving image 4. Extraction of the attached remaining piece by means of a 35 mm Amplatz GooseNeck snare (ev3 Inc.) with a Judkins Right 4 6 Fr guiding catheter. On the right side is the epicardial pacemaker.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Baseline fluoroscopic images of the broken entrapped lead.
Moving image 2. Removal of the proximal end of the lead by combination of a GooseNeck snare and a 0.035 inch Teflon coated Cordis® wire placed in parallel and around the leads to create a loop at the level of the superior caval vein and left subclavian vein.
Moving image 3. Extraction of the attached remaining piece by means of a 35 mm Amplatz GooseNeck snare (ev3 Inc.) with a Judkins Right 4 6 Fr guiding catheter. On the right side is the epicardial pacemaker.
Moving image 4. Extraction of the attached remaining piece by means of a 35 mm Amplatz GooseNeck snare (ev3 Inc.) with a Judkins Right 4 6 Fr guiding catheter. On the right side is the epicardial pacemaker.

