
Abstract
Aims: Pacemaker lead-associated severe tricuspid regurgitation (TR) can lead to right heart failure and poor prognosis. Surgery in these patients carries significant morbidities. We describe a successful treatment of symptomatic severe TR by leadless pacemaker implantation followed by tricuspid valve (TV) repair with the MitraClip NT.
Methods and results: A 71-year-old frail female with poor functional status, chronic atrial fibrillation and permanent pacemaker implantation in 2012 presented with symptomatic moderate-severe mitral regurgitation (MR) and severe TR with the pacemaker lead as the culprit. She was deemed extreme risk for double valve surgery and, because of her pacemaker dependency, the decision was to stage her interventions first with transcatheter mitral repair, then laser lead extraction and leadless pacemaker implantation to free the TV from tethering, then TV repair. An obstructive LAD lesion was identified and treated during mitral repair with the MitraClip NT. The Micra leadless pacemaker implantation and subsequent TV repair with the MitraClip NT were successful and the patient’s MR improved to mild and TR to moderate, respectively.
Conclusions: We report here a first successful transcatheter strategy to treat lead-associated severe TR by leadless pacemaker and MitraClip. Removing the pacemaker lead relieved leaflet tethering and improved the reparability of the TV.
Abbreviations
ASD: atrial septal defect
CDS: catheter delivery system
GDS: guide delivery system
LAD: left anterior descending
LAO: left anterior oblique
MR: mitral regurgitation
NYHA: New York Heart Association
PCI: percutaneous coronary intervention
RAO: right anterior oblique
TOE: transoesophageal echocardiography
TR: tricuspid regurgitation
TTE: transthoracic echocardiography
Introduction
Pacemaker lead-associated tricuspid regurgitation (TR) is not uncommon and can lead to progressive liver congestion and peripheral oedema, heart failure and death1. Tricuspid valve surgery carries significant mortality and morbidities. Recent US approval of the Micra leadless pacemaker (Micra™ Transcatheter Pacing System; Medtronic Inc., Minneapolis, MN, USA), and the emergence of transcatheter tricuspid repair, may offer a less invasive alternative2,3. We describe a first successful percutaneous treatment of symptomatic severe TR due to pacemaker lead interference, by laser extraction and Micra leadless pacemaker implantation, followed by tricuspid valve repair with the new-generation MitraClip® NT (Abbott Vascular [Structural Heart], Santa Clara, CA, USA).
Case description
A 71-year-old frail female with poor functional status, chronic atrial fibrillation and permanent pacemaker implantation in 2012 presented with symptomatic moderate-severe mitral regurgitation (MR) and severe TR. She had advanced NYHA Class III-IV symptoms with significant dyspnoea on exertion and peripheral oedema. Transoesophageal echocardiography (TOE) revealed prolapse of the posterior mitral valve leaflet and TR due to septal leaflet tethering with the ventricular lead (Figure 1A, Figure 1B). Left ventricular function was preserved but the right ventricle was dilated. She was deemed extreme risk for double valve surgery due to her comorbidities and frailty, and a transcatheter solution was considered. Because of pacemaker dependency, the decision was to stage her interventions first with transcatheter mitral repair, then laser lead extraction and leadless pacemaker implantation to free the tricuspid valve from tethering, and then transcatheter tricuspid repair if necessary.
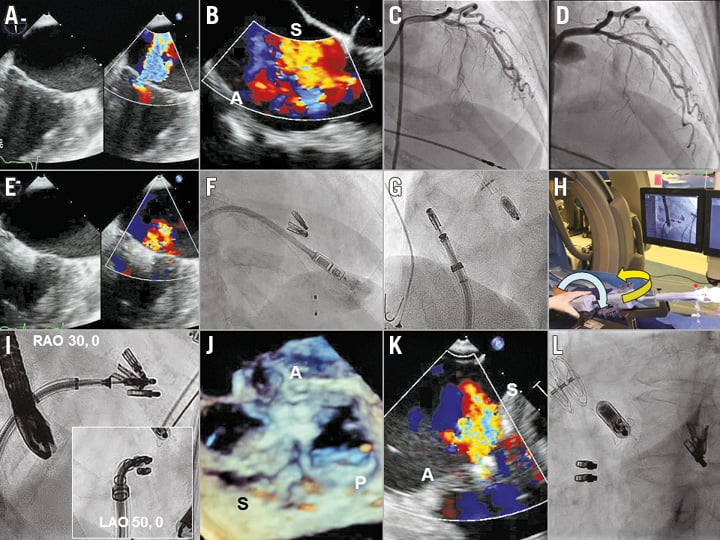
Figure 1. Transcatheter tricuspid valve repair with MitraClip NT after Micra pacemaker implantation. Baseline moderate-severe mitral and severe tricuspid regurgitation (A, B). Incidental finding of LAD stenosis and after PCI (C, D). Mild MR after two MitraClips (E). Micra implantation after laser lead extraction (F). Modified Munich approach enabled CDS straddling to the GDS and steering of the CDS to the tricuspid valve (G, H). Fluoroscopic guidance facilitates MitraClip orientation, positioning and deployment (I). TOE showed the final result with two clips placed across anterior and septal leaflets (J), improving to moderate TR (K), and final fluoroscopic view after the procedure (L). A: anterior; LAO: left anterior oblique; P: posterior; RAO: right anterior oblique; S: septal
Mitral valve repair with the MitraClip NT was successfully performed during which an obstructive mid-left anterior descending artery (LAD) lesion was treated with percutaneous coronary intervention (PCI) (XIENCE Alpine™; Abbott Vascular) (Figure 1C, Figure 1D). Two clips were placed at the A2/P2 segments, the MR was reduced to mild and the atrial septal defect (ASD) was closed with a GORE® CARDIOFORM device (W.L. Gore & Associates, Flagstaff, AZ, USA) (Figure 1E). The patient was discharged home uneventfully on day two. However, she remained symptomatic, and transthoracic echocardiography (TTE) at 30 days showed mild MR and severe TR. The patient then underwent a laser lead extraction and percutaneous transfemoral Micra leadless pacemaker implantation (Figure 1F). Unfortunately, the TR remained severe.
Two weeks later, she returned for tricuspid valve repair with the MitraClip NT. Intraoperative TOE showed that the TR originated between the anteroseptal and posteroseptal leaflets. Under fluoroscopic and TOE guidance, we used the modified Munich approach by miskeying the clip delivery system (CDS) counterclockwise 90 degrees when inserting into the guide delivery system (GDS). This enabled straddling the CDS against the GDS to optimise manoeuvrability of the NT system (Figure 1G). Turning the GDS clockwise by 180 degrees with the +/- knob facing downward, and the A knob by >180 degrees steered the MitraClip towards the tricuspid valve (Figure 1H). Minor turns of the GDS and the A knob effectively oriented the MitraClip towards the grasp plane aligning anterior and septal leaflets. Using the left anterior oblique (LAO) projection for clip orientation and right anterior oblique (RAO) projection for clip positioning and delivery, two MitraClips were successfully deployed grasping the anterior and septal leaflets, reducing the TR to moderate in severity (Figure 1I-Figure 1L). The patient was discharged home uneventfully on day two and currently remains well with NYHA Class I-II symptoms and reduced diuretic requirement. TTE at 30 days showed mild MR and moderate TR.
Discussion
Symptomatic severe TR carries a dismal prognosis with few treatment options. Surgery carries significant risks and transcatheter treatment remains in its infancy with limited experience. One of the most vexing problems in TR is associated with pacemaker lead interference. These patients are almost always pacemaker dependent and lead extraction to attempt to improve TR may not be successful. Transcatheter options to address lead-associated TR are also limited, due to potential interference of the pacing lead during device implantation4.
Recent approval of the Micra leadless pacemaker offers a potential solution in these patients. As shown in our case, a staged lead extraction and leadless pacemaker implantation, followed by transcatheter tricuspid repair, can lead to significant TR reduction and clinical improvement. The issue of lead interference is resolved with Micra implantation, providing transcatheter options to address the TR. We used the MitraClip NT in our case given that no other transcatheter tricuspid repair devices were commercially available. Successful experience with MitraClip tricuspid repair has been reported, with >300 cases performed worldwide5. The NT system enables further lowering of the grippers to improve leaflet grasping and is particularly suited for the tricuspid valve, given that the aetiology is usually annular dilatation and leaflet tethering.
We utilised the modified Munich approach for tricuspid clipping because of the improved manoeuvrability associated with the CDS straddling against the GDS. At the 2016 PCR London Valves, Hausleiter and colleagues presented their series using the modified Munich approach with significant TR reduction and clinical improvement. We adopted the same technique and found that miskeying between the CDS and the GDS easily enabled device straddling and allowed us to perform minute adjustments to the NT system to perform the procedure efficiently and successfully. The anterior and septal leaflets are most easily targeted given the favourable device trajectory and tricuspid valve anatomy.
Other interesting findings in our case were concomitant moderate-severe MR, prompting us first to perform mitral repair with the NT, and the incidental mid-LAD lesion leading to simultaneous PCI. Given that the patient also had severe TR and a dilated right ventricle, we closed the ASD to eliminate the left to right shunt and right heart volume overload. Interestingly, the MitraClips in the mitral position and the ASD occluder served as useful landmarks to guide positioning and orientation of the MitraClips during our tricuspid repair. Moving echocardiographic and fluoroscopic images of the procedure, in chronological order, can be found in the supplementary data section (Moving image 1-Moving image 8).
Conclusions
Our case demonstrates the first successful staged Micra implantation and MitraClip NT tricuspid valve repair to manage lead-associated severe TR. We believe that the leadless pacemaker will provide these inoperable patients with options for transcatheter tricuspid repair to improve their symptoms, quality of life and prognosis.
| Impact on daily practice Staged percutaneous leadless pacemaker implantation and tricuspid valve repair with the MitraClip NT is feasible and effective in the treatment of high-risk patients with symptomatic severe tricuspid regurgitation associated with a pacing lead. The modified Munich approach to tricuspid clipping may serve as an alternative to the classic approach. Using both fluoroscopic and echocardiographic guidance optimises procedural success. |
Conflict of interest statement
G. Tang is a consultant, advisory board member and faculty trainer for Abbott Structural Heart. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Bicommissural view. Baseline MR.
Moving image 2. Four-chamber view. Baseline TR.
Moving image 3. Bicommissural view. Mild MR after MitraClip mitral repair.
Moving image 4. Micra leadless pacemaker implantation.
Moving image 5. Fluoroscopic view of MitraClip NT steering to tricuspid valve using the modified Munich approach.
Moving image 6. Fluoroscopic view showing LAO to RAO orientation of the MitraClip NT CDS at the tricuspid valve.
Moving image 7. TOE depicting final result after tricuspid clipping.
Moving image 8. Four-chamber view. Moderate TR after tricuspid repair.
Supplementary data
To read the full content of this article, please download the PDF.
Bicommissural view. Baseline MR.
Four-chamber view. Baseline TR.
Bicommissural view. Mild MR after MitraClip mitral repair.
Micra leadless pacemaker implantation.
Fluoroscopic view of MitraClip NT steering to tricuspid valve using the modified Munich approach.
Fluoroscopic view showing LAO to RAO orientation of the MitraClip NT CDS at the tricuspid valve.
TOE depicting final result after tricuspid clipping.
Four-chamber view. Moderate TR after tricuspid repair.

