
CASE SUMMARY
BACKGROUND: A 21-year-old man with a history of atrial septal defect (ASD) and ventricular septal defect (VSD) repair and tricuspid valve (TV) replacement with size 22 mm aortic homograft presented complaining of severe shortness of breath. Clubbing and cyanosis of the fingers were noted. SpO2 on room air was 80-84%.
INVESTIGATION: Physical examination, electrocardiogram, chest X-ray, transthoracic echocardiography, CT scan, right cardiac catheterisation.
DIAGNOSIS: Persistent left superior vena cava draining into the left atrium through an unroofed coronary sinus, severe stenosis and regurgitation of homograft in the tricuspid position.
MANAGEMENT: Percutaneous closure of left superior vena cava draining into the left atrium through an unroofed coronary sinus with concomitant percutaneous implantation of a Melody valve in the tricuspid position.
KEYWORDS: persistent left superior vena cava, unroofed coronary sinus, Ebsteinoid tricuspid valve
PRESENTATION OF THE CASE
A 21-year-old male known to have atrial septal defect (ASD), ventricular septal defect (VSD) and an Ebsteinoid tricuspid valve (TV) underwent ASD and VSD repair and TV replacement with a size 22 mm aortic homograft when he was four years old.
The patient had been followed up in our institution, developing progressive TV stenosis and regurgitation. In 2013 he underwent a Bruce protocol exercise test and he was able to achieve 13.1 METS (10 min 55”). He had started complaining of shortness of breath over the past two years. The patient denied any history of loss of consciousness, syncope or chest pain.
He presented recently claiming severe shortness of breath. Clubbing and cyanosis of the fingers was present. SpO2 on room air was 80-84%.
ECG showed normal sinus rhythm and right bundle branch block (RBBB). On laboratory examination, red blood cells (7.3×1,012/L) and bilirubin (41.7 µmol/L) were elevated. Chest X-ray demonstrated a mildly enlarged heart size.
Transthoracic echocardiography (TTE) showed normal left ventricular (LV) size and function, a moderately dilated right ventricle (RV) with moderately to severely impaired function; TV homograft presented grade III-IV regurgitation and TV area by pressure half time was 0.7 cm². RV systolic pressure was normal. A persistent left superior vena cava (PLSVC) with unroofed coronary sinus (UCS) was demonstrated with saline echo contrast injection via the left antecubital vein, showing bubbles draining through the left atrium (LA) then passing to the LV before appearing in the right atrium (RA) and RV (Figure 1).
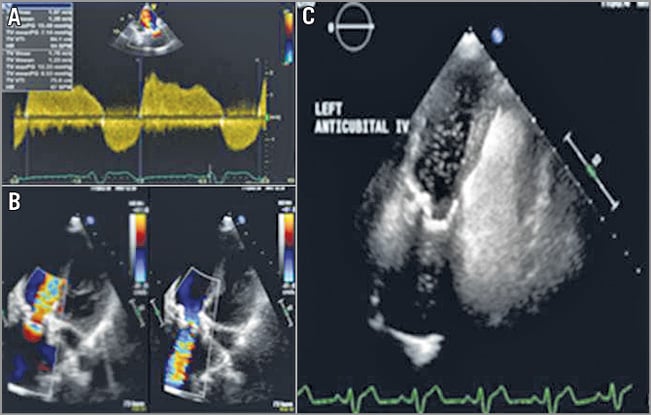
Figure 1. Echocardiographic study. A) TTE modified parasternal long-axis view of the tricuspid valve. Continuous wave Doppler showing severe stenosis of the size 22 mm homograft. B) TTE 2D colour apical four-chamber view showing severe stenosis and regurgitation of the homograft in the tricuspid position. C) TTE apical four-chamber view with left antecubital access showing i.v. saline echo-contrast (bubble study) opacification bubbles going into the left atrium and left ventricle first, then appearing in the right atrium and right ventricle.
A computed tomography (CT) scan showed that the LSVC ran along the left side of the aortic arch then entered posteriorly and beneath the level of the LA appendage following the course of the UCS with direct continuity with the LA instead (Figure 2).
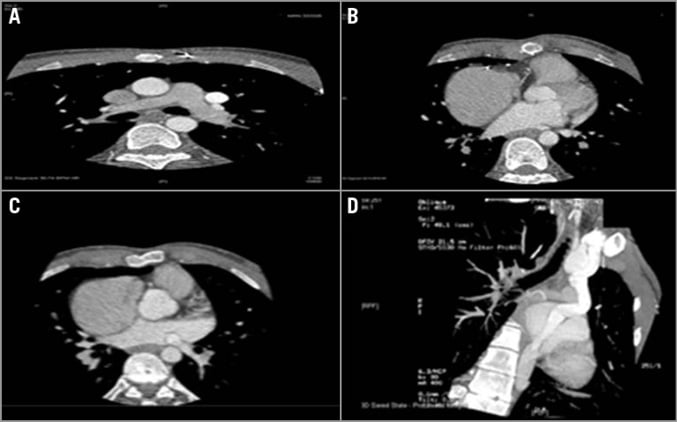
Figure 2. Computed tomography study. A) CT scan retrospective ECG gated at the level of the main pulmonary artery (MPA) with the PLSVC seen running along the left side of the MPA. B) & C) The LSVC seen running along the left side and behind the LA appendage and the LA. D) Multiplanar reformatting (MPR) showing the PLSVC dilated and draining into the RA with UCS.
A right cardiac catheterisation demonstrated Qp:Qs=0.75:1, pulmonary vascular resistance of 4.0 WU. RV pressure was 20% systemic. Simultaneous pressure recording of RA and RV showed 7 mmHg gradients. Innominate injection showed the LSVC draining into the UCS (Figure 3A). An RV angiogram showed severely reduced systolic function and severe TR (Figure 3B).
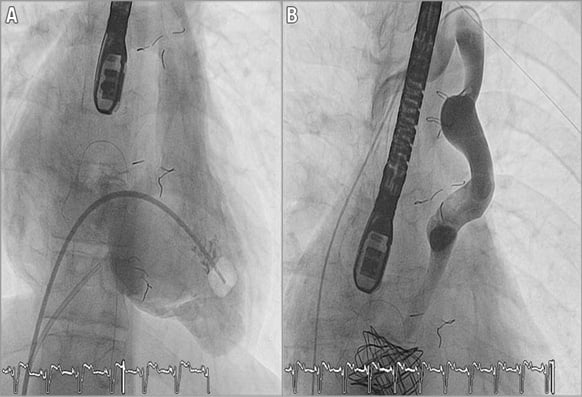
Figure 3. Angiographic evaluation. A) Anteroposterior (AP) view of RV systole showing significant regurgitation of the homograft in the tricuspid position. B) AP view of the LSVC to the UCS. Innominate vein connecting the LSVC to the RSVC.
A PLSVC draining into the LA, directly or through an UCS, is a rare finding1 and, to our knowledge, a case of UCS associated with an Ebsteinoid TV has never been described before.
The clinical presentation was largely determined by the size of the defect and the degree of shunting. The patient was initially completely asymptomatic. Subsequently, with the development of progressive TV stenosis and regurgitation, the RA pressure increased, leading to cyanosis and symptoms of right-sided heart failure.
What would be the best treatment option for this patient?
How would I treat?
THE INVITED EXPERT’S OPINION

The indication for intervention in this case is clear: 1) manage the persistent left superior vena cava (LSVC) which, associated with the unroofed coronary sinus, is the cause of the significant desaturation; 2) take care of the failing tricuspid bioprosthesis because this patient has been doing well, completely asymptomatic for a long time, until TV stenosis and regurgitation increased the RA pressure leading to cyanosis and symptoms of right-sided heart failure.
Persistent LSVC is the most common congenital thoracic venous anomaly. The most common subtype of LSVC results in the presence of both left and right SVCs. A bridging innominate vein may (35%) or may not (65%) be present2. It is always mandatory to identify collateral vessels to the right superior vena cava (RSVC) prior to consideration of percutaneous closure of an LSVC3.
In this patient the diagnostic angiogram was carried out injecting into the innominate vein. There are many devices that we may use to occlude an LSVC; the AMPLATZER vascular device family (St. Jude Medical/Abbott) is easily conformable and has a wide range of shapes and sizes.
Securing the prosthesis with a snare catheter before the final release might minimise the risks of device displacement/embolisation, anticipate the final position of the device, and rule out any device-related coronary sinus obstruction.
The second step of the procedure should be a valve-in-valve approach to the failed tricuspid bioprosthesis. I suggest a femoral access - easier to manage by the interventionist than the jugular approach. A balloon interrogation would be indicated in this specific case in order to determine the actual inner diameter of the prosthesis, and to maximise orifice area (the homograft does not have a metallic cage). Transoesophageal monitoring may be used during the procedure. A 0.035” super stiff guidewire may be placed distally into the right ventricle; using this position, we have less system stability but a better coaxial position of the delivery system. I would use a Melody™ valve (Medtronic, Minneapolis, MN, USA). The outcomes do not differ significantly according to valve type: Melody and SAPIEN (Edwards Lifesciences, Irvine, CA, USA) valves differ in a number of ways but, for the purpose of this application, one of the most important factors is the range of available sizes. Patients with a smaller surgical TV prosthesis are more likely to receive a Melody valve but, among patients with a large surgical valve (i.e., ≥29 mm), SAPIEN valves are indicated4.
Another issue that suggests to me that a Melody valve should be used in this specific case is that the SAPIEN family of valves is shorter and may be more challenging to align coaxially and seat appropriately in the frame of a homograft without a good landmark during valve implantation. I would suggest avoiding pre-stenting this bioprosthesis because it may reduce the internal diameter. A final post-dilatation may conclude the procedure.
Conflict of interest statement
The author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERT’S OPINION
The initial approach to this case (when the patient was a four-year-old) was unusual and a more standard approach at that stage would have been a tricuspid repair (cone type, Danielson or otherwise) and ASD and VSD closure. If there was a bridging vein from the left SVC to the right SVC, which it looks like there was on the angio provided, then the left SVC could have been ligated outside the heart and the unroofed coronary sinus closed off to the left atrium at the time of ASD closure. This may have avoided all of the subsequent problems.
However, with the case now in a 21-year-old, presumably average weight 70 kg adult, the tricuspid homograft is far too small and severely regurgitant, and the stenosis and regurgitation have led to a right to left shunt across the unroofed coronary sinus into the left atrium as the Qp:Qs measurement demonstrates. The patient’s main issues are the following.
1. Cyanosis is related to this shunt. I do not believe that the left SVC to unroofed CS is the main cause of this cyanosis, as the volume load from the left SVC, particularly with a bridging vein, would not be very significant at all to the left atrium. The main shunt is RA to LA via the unroofed CS from higher RA pressures related to the tricuspid valve.
2. The right ventricular dysfunction is probably related to chronic tricuspid stenosis and regurgitation, as the RV pressures are normal, suggesting that there are no pulmonary hypertensive concerns.
Therefore, the main consideration comes down to dealing with the tricuspid valve in a way that will provide a long-lasting solution with no residual stenosis or regurgitation for a very young adult. I would have major concerns with stenting the homograft with a Melody valve as this will not provide a good long-term solution and will probably leave a residual inflow gradient as, even if the homograft can now be stretched to 22 mm (its original size) with the Melody, this is too small an inflow for an average adult patient for the long term.
My preferred option would be to replace the tricuspid homograft with a tissue mitral valve of better orifice, i.e., 27/29 mm, particularly to avoid warfarin for a young man. At the same procedure, the unroofed coronary sinus could be closed at its right atrial side so that coronary sinus blood drains to the left atrium and the left SVC could be ligated outside the heart if there is an adequately sized bridging vein. This would produce an excellent orifice area tricuspid valve for the patient for the future and, if this valve fails in the future, then a valve-in-valve procedure with a better orifice area could be performed, negating the need for further surgery.
I believe, however, that using a Melody valve in the homograft would provide a very short-term result that will need surgical revision in the future and, later still, a valve-in-valve procedure. For these reasons, I do not see what benefits a Melody procedure could offer the patient at this stage and would have opted for a redo surgery, to place a larger bisoprosthetic valve to avoid patient prosthesis mismatch.
Conflict of interest statement
The author has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Coexistence of UCS and PLSVC might result in right chamber volume overload, in the case of significant left to right shunt, or predispose to life-threatening complications such as cyanosis and paradoxical embolisation1.
Surgical treatment of PLSVC with UCS is challenging and requires the use of prosthetic material to correct its components and associated abnormalities5. In our patient, due to the favourable anatomic arrangement, we thought that a percutaneous procedure was feasible.
Intervention
TRICUSPID VALVE HOMOGRAFT SIZING
A 0.035” 260 cm-long Amplatz Super Stiff™ (Boston Scientific, Marlborough, MA, USA) 1 cm tip wire was placed distal to the left pulmonary artery. A 20×40 mm Cristal balloon (Balt Extrusion, Montmorency, France) was tracked and positioned on TV, inflated at maximum 5 atm pressure.
STENTING
Using a 13 Fr Check-Flo® (Cook Medical, Bloomington, IN, USA) over the 0.035” 260 cm Amplatz Super Stiff 1 cm tip guidewire, a 34 mm Covered CP Stent™ (NuMED Inc., Hopkinton, NY, USA), which was mounted on a 20×40 mm BIB® balloon (NuMED Inc.), was positioned at the homograft site and inflated at maximum 6 atm pressure twice. Stent dilatation was upgraded sequentially with a high-pressure 20×20 mm Atlas® balloon (Bard PV, Tempe, AZ, USA), inflated at maximum 15 atm pressure twice, and a 22×20 mm Atlas balloon at maximum pressure of 12 atm.
TRICUSPID VALVE IMPLANTATION
A 22 mm Melody tricuspid valve (Medtronic) was prepared and crimped onto the 22 mm Ensemble® Delivery System (Medtronic). A 1 cm tip wire was inserted over the 0.035” 260 cm-long Amplatz Super Stiff wire and positioned next to the stent prior to successful deployment. The valve was successfully inflated at maximum pressure (Figure 4A).
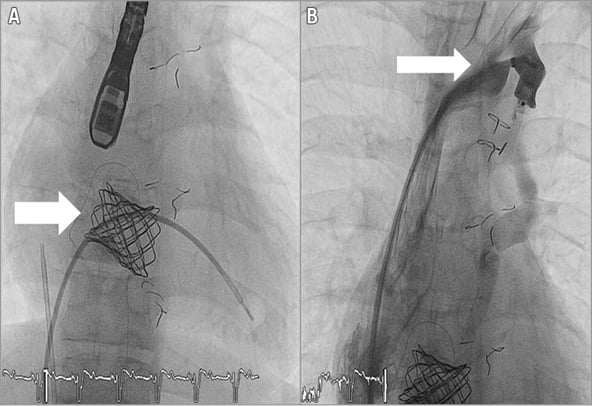
Figure 4. Melody valve implantation. A) Anteroposterior (AP) view of RV systole after Melody valve implantation (arrow). B) AP view of the LSVC to the UCS. AMPLATZER device seen occluding the LSVC (arrow).
LSVC OCCLUSION
Using a 7 Fr AMPLATZER® Occluder System (St. Jude Medical) over the 0.035” 260 cm-long Amplatz Super Stiff 1 cm tip guidewire, a 12×5 mm AMPLATZER® Vascular Plug III (St. Jude Medical) was positioned and deployed successfully (Figure 4B).
The early postoperative course showed brief runs of supraventricular tachycardia, treated with amiodarone. After three months, on physical examination the patient was looking well, was asymptomatic, his heart rate was 94 bpm, blood pressure 113/74 mmHg, and SpO2 was 96% on room air. Systemic examination was unremarkable. He was only on 5 mg enalapril. TTE was carried out showing that the Melody valve was well seated and functioning well with no significant stenosis or regurgitation. Injection of contrast using the left antecubital vein documented no bubbles in the LA (Figure 5).
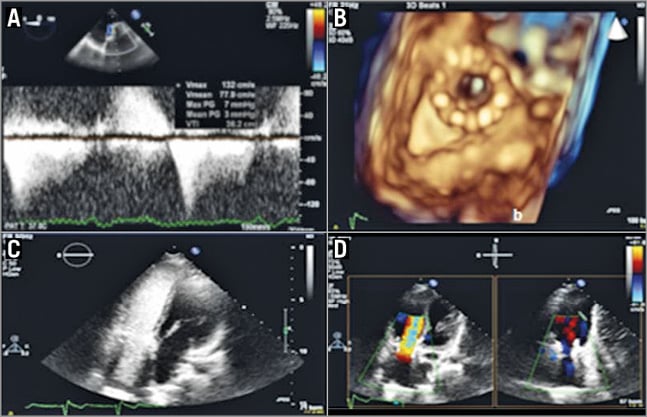
Figure 5. Post-procedural echocardiographic evaluation. A) Transoesophageal (TEE) mid-oesophageal view of the implanted Melody valve in the tricuspid position. Continuous wave Doppler showing no significant gradient across the Melody valve. B) TEE 3D zoom imaging en face view of the Melody valve in the tricuspid position. C) TTE apical four-chamber view with left antecubital access showing i.v. saline echo-contrast opacification bubbles going directly into the right atrium. D) TTE 2D colour apical four-chamber view showing no significant regurgitation across the implanted Melody valve.
Discussion
Transcatheter closure of UCS has only been described twice6,7 as has percutaneous closure of a PLSVC draining into the LA3,8.
This article reports on the first case of percutaneous closure of an LSVC draining into the LA through a UCS with concomitant percutaneous implantation of a Melody valve in the tricuspid position.
To our knowledge, this is the first case of UCS associated with Ebsteinoid TV to be successfully treated with a percutaneous Melody valve and occlusion of the LSVC with an AMPLATZER device.
However, a careful assessment of the local anatomy and flow patterns before and after device implantation should always be mandatory, because this procedure is challenging and carries a higher risk in relation to standard surgical management.
Conflict of interest statement
The authors have no conflicts of interest to declare.

