Although surgical mitral valve repair has been shown to be superior to mitral valve replacement1, the durability of a successful repair is much inferior in patients with functional mitral regurgitation2. With the emergent transcatheter valve technologies over the past ten years, there have been a myriad of technological approaches to treat mitral regurgitation that we classified according to their mechanism of action on the mitral valve3. The indirect annuloplasty devices seek remodelling of the mitral annulus by reshaping the coronary sinus, taking advantage of the anatomical proximity of both structures. The mitral annulus, however, lacks a true “annulus fibrosus” structure and is rather a fibrous-band like tissue that histologically is part of the greater structural system that is directly connected to the cardiac skeleton, connecting the membranous septum, the two trigones and the attachment of the aortic root to the left ventricular muscle4. This atrioventricular structure has a hyperbolic-paraboloid shape to reduce the mechanical strains on the posterior leaflet during systolic valve closure5. The coronary sinus / great cardiac vein (CS/GCV) somewhat parallels this atrioventricular structure, however the distances between those two structures progressively increases over the first 4-5cm of the CS/GCV trajectory and decreases thereafter. Furthermore, these distances are very dynamic during the cardiac cycle with the largest distance found at the end of systole and the smallest at the end of diastole6. In addition, in 86% of patients, there is a coronary artery traveling between the most external venous structures and the deeper fibromuscular structures, with a risk, therefore, of causing external compression on coronary arteries with resultant myocardial ischaemia6. Thus, the placement of stiff devices within the CS/GCV, may not only cause compression of coronary arteries running in between the venous structures and the atrioventricular fibromuscular structure, but also can exert external compression on other structures such as the most posterior non-coronary sinus of Valsalva of aorta (Figure 1).
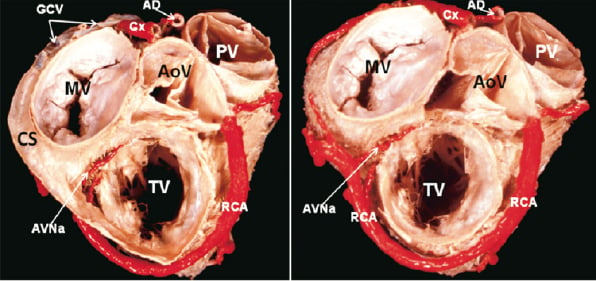
Figure 1. The ventricular base of the heart is viewed from the atrial aspect. Note in A the location of the coronary sinus (CS) in the left atrioventricular groove surrounding the mitral valve (MV). In B the coronary sinus and non-coronay leaflet of the aortic valve (AoV) have been removed to show the dissection of the coronary arteries which were painted in red colour. CS: coronary sinus; MV: mitral valve; TV: tricuspid valve; AoV: aortic valve; PV: pulmonary valve; RCA: right coronary artery; Cx: circumflex artery; GCV: great cardiac vein; AVNa: atrioventricular nodal artery
In this issue of the journal, Van Mieghem NM et al7 describe the technical characteristics of the Viacor PTMA device, a transcatheter indirect annuloplasty device, and they briefly take an early peek at the PTOLEMY-2 clinical experience with this device. They state that out of the 32 patients enrolled, 29 were treated and 24 (75%) had procedural success and only 73% of those showed MR reduction at three months (54% by ITT). These preliminary results are far from optimistic and actually, not much different from the PTOLEMY-1 results8.
Perhaps one of the reasons for the low yield of success is that, the compression of the CS by the stiff rods exerts the force along the vessel trajectory, and this venous structure is further away from the mitral annulus at the level of the P2 scallop. As a matter of fact this distance is largest during the end of systole6 and therefore, further away from the annulus, when the anterior deformation of the posterior segment of the annulus is mostly needed to enhance leaflet coaptation. Thus, the force required at this higher level to effectively cause an anterior displacement of the lower sited annular structure may impose a high stress level on these mostly muscular structures. An effective mitral regurgitation reduction, therefore, may be achieved only at the expenses of higher forces applied above the annulus with a potential increase in the risk of erosion. In this issue of the journal Noble S et al9 describe a fatal erosion of the device through the CS/GCV into the ascending aorta that had not been previously reported. This complication could also be anticipated by understanding the dynamic anatomical relationship of the CS and the most posterior non-coronary sinus of Valsalva of the aorta.
Although attractive in concept, the reality of indirect annuloplasty using the deformation of CS to modulate the shape of the fibromuscular atrioventricular junction to decrease mitral regurgitation faces insurmountable anatomical handicaps to accomplish the goal and poses significant risks of lethal complications. The number of patients, therefore, that potentially could fulfil all the necessary anatomical requirement to be effective and safe in the long run (the lack of any coronary artery running underneath the venous structures and an adequate distance of the CS to the mitral annulus) may be relatively small when considering the large pool of patients with functional mitral regurgitation.
Conflict of interest statement
The authors have no conflict of interest to declare.
References

