- annuloplasty
- coronary sinus
- mitral valve regurgitation
- complications of percutaneous intervention
An 83-year-old farmer was admitted for congestive heart failure. Transthoracic echocardiography (TTE) revealed functional grade 3 mitral regurgitation (MR) and moderately decreased left ventricular ejection fraction (LVEF). A coronary angiogram demonstrated no significant lesions. Despite optimal medical therapy for six months, the patient remained in functional class III and was offered percutaneous mitral valve repair using the experimental Viacor PTMA (percutaneous transvenous mitral annuloplasty) device1 within PTOLEMY-2 protocol. The procedure was performed under general anaesthesia, with fluoroscopic and transesophageal echocardiography guidance. Vascular access was established from the left subclavian vein to the coronary sinus (CS) using standard 8 Fr CS sheath. A PTMA catheter was then advanced over a 0.035” wire past the great cardiac vein into the anterior interventricular vein. Into this catheter, two 120-mm nitinol (nickel-titanium alloy) rods were inserted to apply stiffness selectively to the P2 segment of the mitral valve, in order to reduce the septal-lateral valve dimension, improve leaflet coaptation, and reduce MR. Septal-lateral annulus diameter reduction was achieved (Figure 1) and MR was reduced to grade 1. At day one, he described mild upper abdominal pain, not reported thereafter, and he was discharged at day three after having ruled out pericardial effusion by TTE and device migration by chest radiograph. At day 10, he self-medicated to relieve new onset of back pain. The day after, he was transferred to the emergency room in shock with intense back pain. A contrast induced CT scan showed a large haemothorax and an acute bleeding source on the descending aorta (Figure 2a). In accordance with the patient’s wishes, no emergent surgery was attempted and he died six hours later.
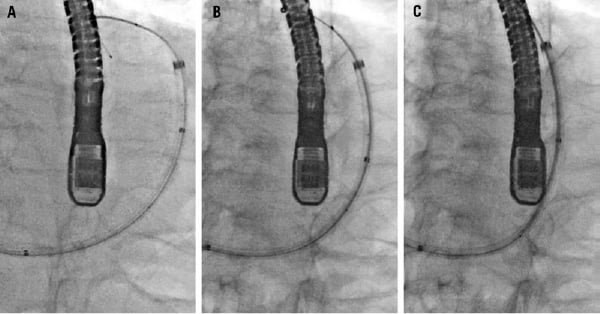
Figure 1. Fluoroscopy series (left anterior oblique 25° and caudal 25° projection) during the procedure showing annulus diameter reduction. (unchanged position of echo probe and patient). A. PTMA catheter located in the coronary sinus over a wire. B. One rod inserted into the catheter. C. Two rods inserted. The septum is located on the left side of the figureand the lateral wall on the right side. The aortic valve is around 11 o’clock on the upper part of the figure.
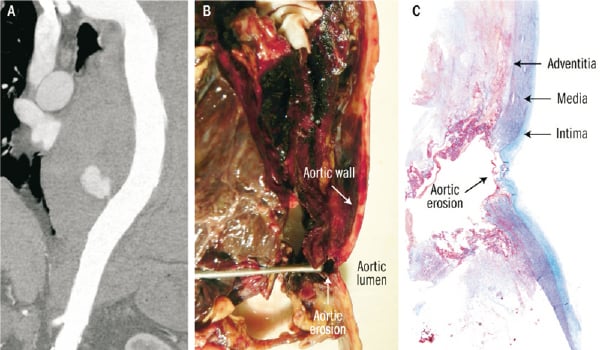
Figure 2. A. Contrast enhanced CT scan showing contrast leak at the descending aorta level (anterior portion) B. Macroscopic view of the aortic wall at the autopsy with the probe showing the erosion (probe inserted through the haematoma between the pericardium and the descending aorta) C. Masson’s trichrome stain of the aortic erosion.
Autopsy showed perforation of the CS at 0.7 cm from the ostium, with subsequent pericardium and descending aorta erosions (Figures 2b-c and 3a-b). PTMA device did not migrate, but its proximal portion was protruding into the CS erosion by 2 cm, which corresponded approximately to the distance between the CS and descending aorta. Microscopic analysis revealed an inflammatory reaction (Figure 3c) not only around the CS and pericardial erosion, but also around the mediastinal haematoma and descending aorta suggesting that the erosion started seven days prior the acute event (aortic leak) leading to death.
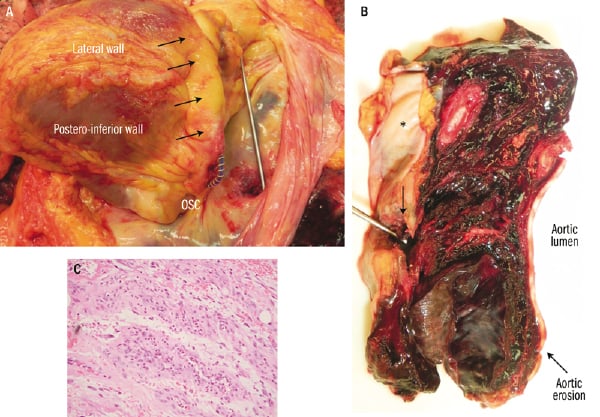
Figure 3. A. Macroscopic view with the pericardium open and the heart reclined (towards the right pleural cavity) showing PTMA device protruding across the coronary sinus erosion. Probe across pericardial erosion. Black arrows showing the coronary sinus. OSC: ostium of coronary sinus. B.View of the haematoma between the pericardium and the descending aorta. Arrow showing the pericardial erosion. *denotes the inner surface of the pericardium. C. Haematoxylin and eosin stain at the level of the mediastinal haematoma showing granulation tissue.
The CS erosion occurred in a bend (Figure 4) where the stiff rods applied an outward tension. The concept of the PTMA device is different from the other CS annuloplasty devices which use the CS to cinch the mitral annulus. Indeed, the nitinol rods of the PTMA device apply pressure over the mid-segment of the mitral annulus, and just proximal and distal to the point of pressure, due to the effect of leverage, they exert an outward tension away from the mitral annulus2.
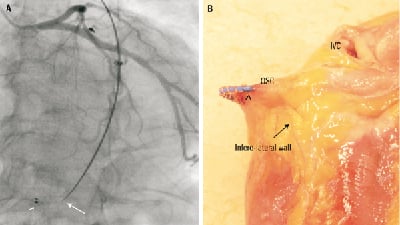
Figure 4. A. Angiography (Left anterior oblique 25° and caudal 23° projection) showing the final position of PTMA device and its relation to left coronary artery. Arrow head showing the fluoroscopic marker positioned at the coronary sinus ostium. Arrow showing the end of the rigid part of the rod with a sharp angulation. B. Macroscopic view showing PTMA device protruding across the coronary sinus erosion. Arrow head showing the fluoroscopic marker. OSC: ostium coronary sinus; IVC: inferior vena cava
Although the CS access was easily established and no leak or CS dissection was observed on final angiograms, we cannot exclude aprocedural injury on the vein wall which might have facilitated CS rupture. Erosion from within the vein was listed among the potential major adverse events, but it had never occurred in prior animal or human implantations. This complication leading to death is device-related and potentially procedure-related.
Conflict of interest statement
S. Noble received research support from Viacor Inc directly related to the conduct of the PTOLEMY-2 study. The other authors have no conflict of interest to declare.

