Case summary
Background: A 69 year old man was admitted with unstable angina (Class IIB). He had a history of chronic renal impairment, diabetes mellitus, hypertension and coronary bypass surgery in 1997 (LIMA graft to the LAD and diagonal branch, saphenous vein grafts to the RCA and first marginal branch of LCx).
Investigation: Coronary angiography
Diagnosis: Unstable angina (Class IIB). Occlusion of the LCx and RCA. Significant stenosis of the venous graft on the marginal branch of LCx. Functionally occluded LIMA on the LAD and diagonal branch. Diffuse disease of the LAD with two significant lesions at the LAD-first diagonal and mid-distal LAD.
Treatment: Revascularisation
Keywords: Post-CABG, plaque prolapse, OCT
How should I treat?
Presentation of the case
A 69 year old man was admitted with unstable angina (Class IIB). He had a history of chronic renal impairment, diabetes mellitus, hypertension and coronary bypass surgery in 1997 (LIMA graft to the LAD and diagonal branch, saphenous vein grafts to the RCA and first marginal branch of LCx). Diagnostic coronary angiogram revealed: occlusion of the proximal LCx and RCA. The LAD was diffusely diseased with a critical bifurcation lesion (Medina: 1, 1, 1) at the LAD-first diagonal with collateral flow to the mid and distal LAD from the diagonal branch via a portion of the LIMA conduit (Figure 1, video 1), a significant stenosis of the venous graft on the marginal branch of LCx (Figure 2, video 2), patent venous graft to the RCA. The mid-distal LAD showed a second significant lesion involving the portion of the LIMA anastomosis receiving blood flow from the diagonal branch (Figure 1). Clinical examination revealed: blood pressure of 140/80 mmHg, normal heart rate and regular gallops. Blood count and chemistry were in the normality range except for a creatinine value of 212 µmol/l. ECG showed sinusal rhythm, right bundle branch block. Echocardiogram showed normal cardiac structures and a normal left ventricular function. The exercise test was sub-maximal and clinically positive, interrupted at 120W because of chest pain. The logistic EuroSCORE was 8.3%.
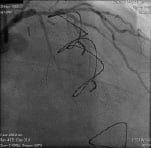
Figure 1. Coronary angiogram image showing a diffusely diseased LAD with critical bifurcation lesion (Medina: 1, 1, 1) on proximal LAD-first diagonal with collateral flow to the mid-distal LAD from the diagonal branch via a portion of the LIMA conduit. The mid-distal LAD shows a second lesion involving the insertion of the LIMA anastomosis.
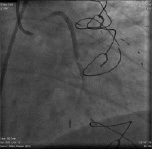
Figure 2. Coronary angiogram image showing a critical lesion of the mid-portion of venous graft on the LCX.
On the basis of the clinical and angiographic findings described above a staged PCI procedure was planned with PCI to the venous graft on the marginal branch first and then two weeks later PCI to the native LAD, unless unstable conditions of the patient required an urgent procedure.
The LCx venous graft was cannulated with a 6 Fr Amplatz Left guiding catheter (Boston Scientific Corp., Nantucket, MA, USA). The lesion in the mid-portion of the venous graft was crossed with a Filterwire 0.014” and the filter was opened distal to the lesion (Figure 3, video 2).
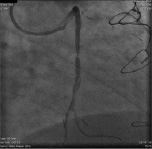
Figure 3. Filter wire insertion and opening of the filter distally to the lesion.
Direct stenting with a 3.0x15 mm (Figure 4) everolimus eluting stent deployed to 20 atm (XIENCE V, Abbott Vascular, Santa Clara, CA, USA). Post-stenting QCA revealed a residual stenosis>20% (Figure 5).
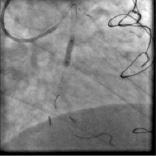
Figure 4. Direct stenting with a 3.0x15 mm everolimus eluting stent deployed to 20 atm (XIENCE V, Abbott Vascular, Santa Clara, CA, USA). No remarkable balloon indentation was angiographically evident.
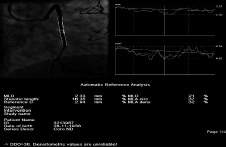
Figure 5. Quantitative coronary angiogram showing a residual stenosis of 21% after direct stenting.
We were faced with the following options: performing a postdilatation on the basis of the angiogram findings; stopping the procedure with the classical “the better is the enemy of good”; or proceed with further intravascular imaging.
How could I treat?
The Invited Experts opinion
Treatment, and especially optimisation, of the results of coronary interventions on saphenous vein grafts (SVG) remain a challenge. In this patient it appears likely that the tight focal lesion located on the mid-shaft of the SVG to the marginal branch actually was the culprit lesion, and explains the patient´s unstable clinical presentation. Many of these old SVG (13 years old in this patient) are diffusely diseased and degenerated indeed, and contain a large burden of friable material. In fact, most of these patients typically present as non-ST elevation myocardial infarction1 although in this case no rise in biomarkers was detected. In this scenario, there is strong evidence supporting the value of adjunctive devices aimed at preventing the effects of distal embolisation2,3. In this patient, the ‘Filterwire system’ was selected. In cases of very tight SVG lesions, advancing and landing this device distal to the lesion may be cumbersome. If this occurs, a conservative balloon dilation (1.5 mm in diameter) may help to facilitate the advancement and distal landing of device and, in our experience, this manoeuvre is not associated with embolic phenomena. However, when no difficulties are encountered – as in the case under discussion – it is clear than direct stenting, after ensuring protection by the ‘Filterwire’, is a much more elegant and secure approach to minimise the risks of distal embolisation2,3.
In this patient the stent was implanted at high pressures with the balloon of the Xience stent. The image displaying balloon inflation appears to show a fully expanded balloon without any appreciable residual waist. However, both issues might be misleading. First, this balloon is moderately compliant, so it remains possible that the stent has not been properly expanded, despite the use of high pressures. Second, the angiographic image of the inflated balloon may actually overestimate the true minimal lumen diameter, especially when only one angiographic projection is presented. Therefore, it remains likely that actually a true under-expanded stent constitutes the underlying substrate explaining the suboptimal angiographic SVG result (MLD=2.33 mm, % diameter stenosis=21%). In this setting, further stent expansion would be indicated. Of interest, optimisation of results may be particularly relevant in SVG lesions taken into consideration the relatively poor long-term outcome obtained in these lesions setting despite the use of drug-eluting stents4,5. Furthermore, suboptimal stent expansion constitutes a clear risk factor for subsequent in-stent restenosis and, more importantly, for stent thrombosis6,7.
Is there any other potential explanation – apart from a poor stent expansion – accounting for the suboptimal results obtained after high pressure balloon inflation? Certainly, tissue prolapse within the stent (either plaque or thrombus) may yield quite similar angiographic images8. This might explain this angiographic appearance after an apparently adequate balloon inflation (no waist detected on the balloon) and use of high pressures (potentially favouring prolapsed of friable material throughout the struts).
In this patient, additional high pressure dilations with a noncompliant balloon may be associated with two distinct problems. In a degenerated old SVG, high pressure dilation may end up in a non-reflow phenomenon despite the “protection” conferred by the ‘Filterwire’. In addition, although fortunately very rare, attempts to further optimise SVG results may also result in vessel perforation9-11. In our experience, perforations in this scenario usually have more favourable outcomes than those occurring in native vessels, because, first, the bleeding may occur outside the pericardium and, second, the post-surgical adherences may confine the effusion. Nevertheless, this remains a potentially devastating complication, typically resulting in cardiac tamponade9-11. Of interest, we have seen some cases where SVG lesions are resistant to high pressures but, in contradistinction to native vessels, this problem usually is not caused by angiographically detected calcification.
Therefore, the performance of an intravascular diagnostic imaging modality should be considered as mandatory to make the appropriate decision6-8. Both intravascular ultrasound (IVUS) and optical coherence tomography (OCT) may be of great value in this setting. IVUS provides a more complete picture of the vessel wall (expansion) but has a lower axial resolution. Alternatively, OCT would provide unique images of plaque or thrombus prolapse, but may lack enough penetration to visualise the complete vessel, especially in large SVG. Further, OCT may differentiate old thrombus (fibrin rich, causing shadowing) from fresh thrombus (platelet reach, allowing light penetration). Currently, however, an accurate differentiation between prolapsing tissue and thrombus within the stent remains elusive. If the stent is indeed under-expanded the case for a further dilation at high pressures (22-24 atm) using a non-compliant balloon is clear. Alternatively, if the stent is well expanded and protruding material is responsible to the suboptimal result aggressive balloon dilations should be avoided. Instead, in this situation we will favour the use of IIbIIIa inhibitors even though its efficacy in SVG is always called into question.
How could I treat?
The Invited Experts opinion
In many centres implantation of bare metal coronary stents (BMS) is the preferred modality for aorto-coronary saphenous vein bypass graft (SVG) lesions, despite the inherent risk for in-stent restenosis. Drug-eluting stents (DES) seemed a promising solution, but conflicting data raised doubts on their efficacy and safety in SVGs. However, in this case we agree with the choice of DES implantation because of the very high risk of restenosis that such a lesion conveys.
As optimal stent implantation1,2 is mandatory to reduce both thrombosis and restenosis in saphenous vein graft stenting, in this complex anatomical subset we tend to adopt an interventional strategy based on intra-vascular imaging. In this case, that shows a sub-optimal QCA evaluation, we would have proceeded with the novel frequency domain Optical Coherence Tomography (FD-OCT) (C7-XR system, LightLab Imaging, MA, USA) assessment.
The new FD-OCT technology permits fast and safe evaluation of stented segment by simply flushing a small amount of contrast (10-14 ml) through the guiding catheter at a pull-back speed up to 20 mm/sec. Like IVUS, OCT enables measurements of reference vessel diameter, minimal stent area and additionally, due to its excellent resolution, is even able to show stent struts malapposition, in-stent plaque protrusion and edge dissection.3-5
In this context FD-OCT imaging would easily tell us if a stent post-dilatation needs really to be done. FD-OCT use can avoid unnecessary post-dilatation in SVG stent, that may be associated with an high risk of distal plaque embolisation, leading to important clinical consequences. The fact that embolic protection device had been removed by operators is an additional reason to proceed with caution.
The moderate amount of contrast used encourages FD-OCT use even in patients with renal insufficiency.
How did I treat?
Actual treatment and management of the case
A coronary optical coherence tomography (OCT) was performed. A 0.019” image wire (LightLab, Boston, MA, USA) was introduced with the distal spring tip 1.5 cm distally to the distal stent edge. Blood clearance was obtained by Visipaque at 37°C flushed through the guiding catheter using automated pump during automated pullback at 3 mm/sec.
The OCT showed a well-apposed stent with mild plaque prolapse (Figure 6) in the stent extending less than 45° from 1 to 3 o’clock, and behind the stent a signal-poor plaque was evident from 2 to 4 o’clock with not clearly defined margins. At this stage, it was decided to keep the angiographic result in order to prevent the shift of the fibro-lipidic plaque and/or dissection that could have occurred during post-dilatation.
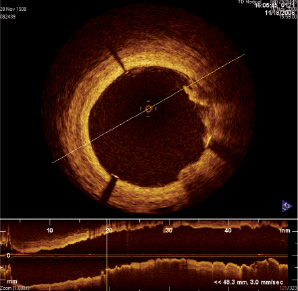
Figure 6. OCT image showing a well-apposed stent with mild plaque prolapse in the stent extending less than 45° from 1 to 3 o’clock and behind the stent a signal-poor plaque was evident from 2 to 4 o’clock with not clearly defined margins.
Discussion points
Evaluation of acute result post-stenting by OCT
This case is a good example of the OCT application in the evaluation of acute result post-stenting. This light-based imaging modality with a high resolution (~10 µm) allows an accurate qualitative assessment of coronary anatomy and plaque composition.1-4
OCT can provide potential advantages over IVUS both in imaging the acute results of PCI with a higher detection of dissection, incomplete stent deployment and tissue prolapse.5,6 The plaque prolapse is characterised by an intraluminal tissue extrusion through the stent struts and it is not always easily detectable using IVUS (spatial resolution ~150 µm). The incidence of plaque prolapse in previous IVUS studies varies from 16-27%7-9. Previous intravascular ultrasound studies have shown that plaque prolapse can contribute to stent thrombosis.10-12 The ability of OCT imaging to identify even a mild prolapse raises some concerns about the necessity to quantify and determine the clinical relevance of plaque prolaps as assessed by OCT.
In this case, OCT showed clearly that all stent struts were well apposed to the vessel wall, but behind the stent a fibro-lipidic plaque was present. A merely angio-guided procedure would have lead to post-dilation of the saphenous graft stent with possible plaque shift and/or dissection of the friable graft wall. Moreover, Hong et al7 have shown that minor plaque prolasee (<25% of the stent CSA) is not associated with higher incidence of late angiographic restenosis at six month follow-up. Therefore, the decision to avoid post-dilatation after the OCT findings seems to be justified. Anyway, further prospective OCT studies are needed to address the consequences of plaque prolapse, as defined by optical coherence tomography, on the early and late clinical outcome after PCI.
Online data supplement

