Abstract
Aims: Pre- and post-interventional optical coherence tomography (OCT) assessment of degenerated saphenous vein grafts (SVG) treated with implantation of pericardium covered stents.
Percutaneous treatment of SVG represents one of the major challenges of current percutaneous coronary interventions (PCI). Artificial membrane-covered stents have failed to show additional benefit over conventional stents.
Methods and results: Six cases of PCI of de novo lesions in degenerated SVGs were successfully treated with a novel pericardium covered stent (PCS). Successful deployment was achieved in all cases. Large emboli were retrieved in a distal filter in one case with a long degenerated lesion. Pre- and post- interventional OCT was performed to assess the lesion characteristics and vessel diameter before stenting and the pericardium layer integrity, strut apposition and presence of plaque prolapse after stenting. In order to better understand the OCT images, three PCS of different diameters were deployed in silicone tubes of 700 µm thickness wall with inner tube diameter matching the stent diameter. OCT was repeated after spreading a thin layer of gel inside the tube, mimicking the toothpaste-like plaque observed in SVG. In vivo and in vitro OCT images excluded the presence of plaque prolapse in all but one case and detected a characteristic pattern with bulging of the pericardium between struts, possibly due to trapping of soft intraluminal plaque (or gel) behind the pericardial layer
Conclusions: These cases offer insight into the mechanism of protection against distal embolisation, elucidated by the appearance of these stents after deployment in vivo and in vitro.
Abbreviations
BMS: bare metal stent
CABG: coronary artery bypass graft surgery
CCS: Canadian Cardiovascular Society
CK-MB: Creatine kinase MB isoenzyme
DES: drug eluting stent
EPD: embolic protection devices
LAD: left anterior descending artery
LIMA: left internal mammary artery
MACE: major adverse cardiac event
MI: myocardial infarction
MLCSA: minimal lumen cross section area
MLD: minimal lumen diameter
OCT: optical coherence tomography
OM: obtuse marginal branch
PCI: percutaneous coronary intervention
PCS: pericardium covered stent
PTFE: polytetrafluoroethylene
SES: sirolimus eluting stent
SVG: saphenous vein graft
TIMI: Thrombolysis in Myocardial Infarction
TVR: target vessel revascularisation
Introduction
Within 10 years after coronary artery bypass graft surgery (CABG), saphenous vein graft (SVG) stenosis or occlusion is observed in more than 50% of cases1. Percutaneous coronary intervention (PCI) of SVGs carries specific technical challenges and has a 15-20% incidence of major adverse cardiac events (MACE), mainly caused by distal embolisation. Different approaches and adjunctive pharmacological regimens have been studied, but with the exception of distal occlusion balloons and filters2,3 which nearly halved non-Q-wave myocardial infarction (MI) to approximately 10% of patients, none have shown a clear benefit in reducing the incidence of distal embolisation. Pooled analysis from five randomised trials and one registry evaluating distal embolic protection devices (EPDs) in SVG PCI (3,958 patients) showed that angiographic measurements of the volume and linear extent of filling defects are the most powerful predictors of adverse 30-day outcomes after SVG PCI4. In the class with the highest quartile of plaque volume, 30-day MACE rates remained as high as 17.3%, in spite of the use of EPDs. Worse results can be expected when diffusely degenerated or completely occluded SVGs (excluded from the above trials) are treated. Furthermore, distal EPDs can be used only in selected patients without extreme tortuosity or occlusions and with a suitable landing zone for the EPD. A slight reduction of events was observed in a study using proximal balloon occlusion, but the technique is cumbersome and not suitable for ostial or very proximal lesions5. Data from American College of Cardiology-National Cardiovascular Data Registry showed that between January 2004 and March 2006 EPDs were used in less than 25% of 19,546 SVG PCI6, despite class I A recommendations in PCI guidelines7.
We describe six cases of PCI of de novo lesions in degenerated SVG treated with a novel pericardium covered stent (PCS, Over and Under® Pericardium Covered Stent, ITGI Medical Ltd, Or Akiva, Israel), with lesion characteristics before treatment and results after stenting assessed with optical coherence tomography (OCT).
Methods
Patients: Six consecutive patients with degenerated plaques in SVGs with highly irregular contours and intraluminal defects were treated with PCSs. The clinical, angiographic and procedural characteristics of the six patients are reported in Tables 1 and 2.
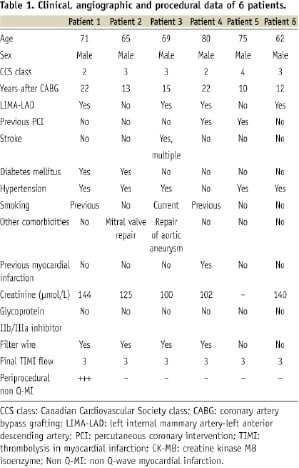
Stent: The PCS consists of a stainless steel stent with 100 µm strut thickness and an uniform 105 µm layer of equine pericardium cylinder made by longitudinal suture. The cylinder is placed over the external body of the stent and under the first and the last elements and then sutured to the edge rows of struts to secure the tissue at both extremities (Figure 1a).
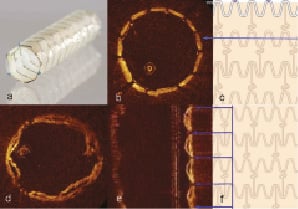
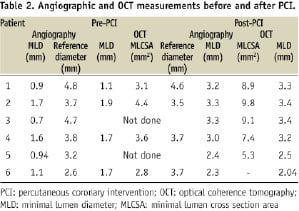
Figure 1. Pericardium covered stent. a. Fully deployed PCS. Please note that the last row of struts is attached to the pericardial membrane and sewn in three points to secure it; b. The OCT image of a 3.0 x 18 mm PCS (across the blue line) deployed within a smooth silicone tube of 3.0 mm diameter shows good apposition of the struts, with the pericardium membrane indistinguishable from the wall of the tube; c. PCS- design features (schematic); d. Same PCS deployed in the 3.0 mm silicone tube after filling it with gel. OCT image showed a hexagonal shape of PCS with bulging of the pericardium into the lumen between struts; e. In the longitudinal view- the gel filled pockets between tube and membrane are more prominent, reaching maximum thickness of 250 µm, a potential useful reservoir for the degenerated friable plaque; f. PCS- design features (schematic).
Because of the semi-compliant balloon used for delivery, during deployment both shoulders of the balloon expand first to avoid dislocation of highly friable plaque, which thereby gets jailed between the vessel wall and the pericardium-covered scaffolding (Figure 3c). The delivery system is theoretically compatible with a 6 Fr guide catheter for the smallest diameters and lengths but 7 Fr guides are preferable. Currently, available PCS diameters are: 3.0, 3.5, 4.0 mm and lengths: 13, 18, 23, 27 mm. The PCS is packaged in a tubular container filled with sterile glutaraldehyde solution. Before implanting PCS, the stent must be rinsed in physiological saline for at least two minutes to remove the preservative prior to use. An important difference with conventional metal stents or membrane covered stents using elastic tissue fabrics is that tearing and disruption of the pericardial covering is likely if the operator exceeds the maximal recommended diameter. Since the balloon is semi-compliant, pressures above 8-10 atmospheres should be avoided during deployment.
OCT imaging technique: OCT is a novel imaging tool for coronary arteries characterised by high intravascular resolution (10-15 µm) but with a limited penetration of 1-1.5 mm in tissue and a maximal depth set at 4 mm. The OCT system used in this study (LightLab Imaging Inc., Westford, MA, USA) consists of a 0.006 inch fibre-optic core that rotates within a non-rotating, 0.019 inch transparent sheath. During image acquisition blood clearing is required, because red blood cells scatter the light. OCT examination was performed using a non-occlusive technique8 with continuous flushing using iodixanol (Visipaque™, GE Healthcare Ltd, Little Chalfont, Buckinghamshire, UK) and motorised pullback at 3 mm/s. Lesion characteristics and vessel diameter before stenting and the pericardium layer integrity, strut apposition and presence of plaque prolapse after stenting were assessed.
In vitro observations: In order to better understand the in vivo OCT images, three PCSs of different diameters were deployed in silicone tubes of 700 µm thickness wall with inner diameters matching the stent diameters. OCT evaluation was prepared before (Figure 1b) and after spreading a thin layer of ultrasound gel (Aquasonic 100; Parker Laboratories Inc., Fairfield, NJ, USA) inside the tube. (Figure 1d). The cross section images showed a good apposition of the PCS to the tube and the pericardium bulging into the lumen between struts (Figures 1d,e), an appearance similar to the in vivo images (Figure 3, III).
Case 1
Angiography showed a diffusely degenerated SVG to an obtuse marginal branch (OM) with highly irregular contours and multiple intraluminal filling defects in different views, highly suggestive of thrombus (Figure 2a). OCT showed protruding plaque with irregular contours, but no material with the characteristics described by Kume at al9 as pathognomic for red or white thrombus (Figure 2 III-IV).
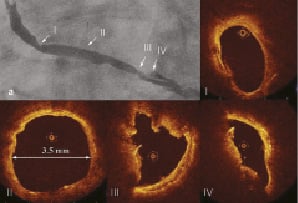
Figure 2. Degenerated SVG lesion. a. Tandem lesion in SVG to OM. The most severe and complex lesion is located in the mid-part of the SVG. OCT images showed: I. Eccentric stenosis with smooth borders of vessel wall; II. Reference segment with mild intimal thickening. The precise measurements of the vessel diameter guide selection of the PCS size20; III and IV. Lesion with a smooth contour and intimal thickening only in a small segment of the vessel circumference, extremely irregular, dishomogenous plaque reduces the minimal lumen area. There are no features typical for red or white thrombus and no clear visualisation of cholesterol clefts or macrophages.
After insertion of a filter wire (FilterWire EZ™, Boston Scientific, Natick, MA, USA), three PCSs (4 x 23 mm; 3.5x 18 mm; 3.5 x 23 mm) were directly implanted without predilatation at 14 atmospheres, leaving generous margins beyond the segment of degeneration, while a MGuard™ mesh covered stent (Inspire-MD, Tel Aviv, Israel) was implanted in the more regular, concentric, fibrous lesion near the ostium of SVG. In the mid-portion of the three deployed PCSs, a persistent short filling defect was observed at angiography (Figure 3a) and confirmed with OCT to have the same characteristics of the pre-existing plaque (Figure 3 I). After an unsuccessful attempt to retrieve it using a PRONTO™ thrombectomy catheter (Vascular Solutions, Inc., Minneapolis, MN, USA), a fourth PCS (3.5 x 23 mm) was inserted and slowly deployed, ensuring full expansion of both edges of the balloon before final stent expansion (Figure 3c). There was a good angiographic result with TIMI 3 flow (Figure 3b). The OCT appearance post PCI confirmed good strut apposition without plaque protrusion (Figure 3 II).
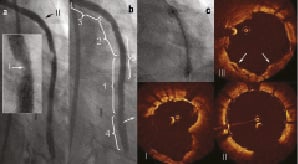
Figure 3. Treatment of SVG. a. Persistent short filling defect in the mid portion of SVG after implantation of 3 PCSs; b. Final angiographic result showing smooth contours after implantation of the 4th PCS; c. Early phase of PCS deployment, with balloon expanded at both extremities, jailing the plaque between the stent and vessel wall; OCT images showed: I. Irregular vessel borders with partially appearing stent struts; II. PCS more proximal to filling defect; III. Pericardium bulging into the vessel lumen, jailing friable plaque behind it (arrows).
Removal of the filter showed multiple yellowish fragments (Figure 4a). Histology of these fragments showed that they were composed mainly of amorphous matrix, fibrin and embedded cholesterol clefts surrounded by macrophages highlighted by immunostaining with CD68 antibodies (Figure 4b-d). Routine postprocedural blood tests revealed a moderate postprocedural rise of myocardial necrosis markers, although the patient did not suffer any chest pain and there were no ECG changes.
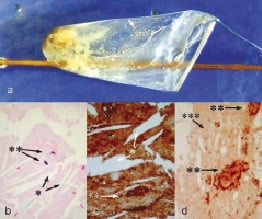
Figure 4. Histological examination. a. Filter wire (FilterWire EZ™, Boston Scientific, Natick, MA, USA) retrieved post-stent deployment, showing multiple yellowish fragments at the end of the procedure; b. Histology showed cholesterol clefts (*) surrounded by macrophages (**) (haematoxylin and eosin staining); c. Immunostaining with antibodies to CD68 delineates better swollen foamy macrophages (**) surrounding the cholesterol clefts (*) embedded in an amorphous, necrotic plaque; d. Immunostaining for fibrinogen highlights fibrin (***) admixed with macrophages (**).
Case 2
Angiography was consistent with a complex, irregular lesion with a filling defect in the proximal segment of a SVG to the left anterior descending artery (LAD) (Figure 5a). Pre-interventional OCT showed a smooth eccentric plaque (Figure 5 I). Direct implantation of 4.0 x 23 mm PCS at 8 atmospheres was performed followed by postdilatation with a 4.0 mm short non-compliant balloon at 16 atmospheres. There was a good angiographic result with TIMI 3 flow (Figure 5b). OCT image confirmed good apposition of the PCS with exception at the proximal edge where focal underexpansion was visible (Figure 5 III).
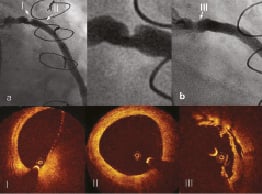
Figure 5. Lesion in SVG. a. Short eccentric lesion with highly irregular contour in proximal segment of SVG to LAD; b. Final angiographic result. Despite aggressive postdilatation up to 16 atm. with non-compliant balloon of 4.0 mm in diameter after PCS deployment focal underexpansion is obvious I. OCT image showed an eccentric lesion; II. Reference vessel segment with a regular lumen 3.5 mm in diameter; III. OCT image confirmed focal underexpansion at the proximal edge of PCS.
Case 3
A proximal complex severe stenosis of a SVG to OM (Figure 6a) was predilated with a 2.0, 2.5 and 3.0 mm non-compliant balloon, advanced over a filter wire (FilterWire EZ™, Boston Scientific, Natick, MA, USA). The balloon expanded well, but because of extreme wall recoil, two attempts of advancing a 4.0 x 27 mm PCS had to be aborted. Despite an 8 Fr Amplatz left 1 guide catheter, two buddy wires including a Choice PT extra support and further predilatation, acute recoil always prevented passage of the PCS. A 4.0 x 27 mm PCS could be advanced only after implantation of a Vision stent (4.0 x 12 mm) to prevent acute recoil. After postdilatation with a 4.0 mm noncompliant balloon, there was a good final angiographic result with TIMI 3 flow (Figure 6b). Final OCT confirmed good strut apposition (Figure 6c).
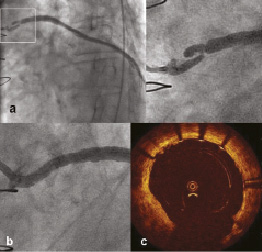
Figure 6. Lesion in SVG. a. Complex severe stenosis at the very proximal part of SVG to OM; b. Final angiographic result; c. Good strut apposition in OCT image.
Case 4
Angiography revealed proximal eccentric lesion in SVG to OM (Figure 7a). Pre-interventional new generation of OCT: Optical frequency-domain imaging (OFDI) showed smooth lesion borders along major part of the circumferential contour and deep fissure. MLA measured 3.6 mm2 (Figure 7 I). Vessel diameter of healthy looking segment just distal to the lesion measured by OFDI was 3.7 mm (Figure 7 II). Direct implantation of 4.0 x 13 mm PCS at 10 atmospheres was performed. During stent deployment there was visible balloon modelling over the resistant lesion (Figure 7b). Postdilatation was performed with a 4.0 mm non-compliant balloon at 19 atmospheres (Figure 7c) with good final angiographic result (Figure 7d). Post-interventional OFDI confirmed good strut apposition (Figure 7 III) with MLA of 7.4 mm2 and without plaque protrusion.
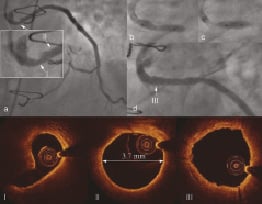
Figure 7. Treatment of SVG to OM. a. Lesion located in the proximal in the proximal segment of the graft; I) OFDI at the level of MLD showed smooth circumferential contour with deep fissure; II) Vessel diameter of healthy looking segment just distal to the lesion measured by OFDI was 3.7 mm; b. Visible modelling of the stent balloon during deployment over the resisting plaque; c. Postdilatation with non-compliant balloon; d. Final angiographic result; III) Well apposed stent struts without protruding plaque.
Case 5
Angiography of a 10-year-old SVG to LAD demonstrated recurrent atherosclerotic disease in the proximal segment of the graft. Two years ago this was treated with drug eluting stent (DES)-TAXUS, but in-stent restenosis occurred. OCT showed a minimal lumen diameter (MLD) of 0.94 mm. After predilatation with a non-compliant 3.0 and 3.5 mm balloon (Mercury NC, Abbott, Abbott Park, IL, USA) PCS (3.5 x 20 mm) was implanted at 10 atmospheres. After additional postdilatation with the same 3.5 mm balloon MLD increased to 2.5 mm (by OCT). OCT demonstrated no plaque protrusion but some underdeployment due the multiple layers of stent struts and the inability to maximally dilate with non-compliant balloon to 20 atm.
Case 6
Angiography revealed a significant lesion between the first and second anastomosis of a jump SVG to OM and posterolateral branch. Direct stenting with a 2.5 x 20 mm PCS (deployed at 10 atm) showed a good angiographic result with a residual diameter stenosis of 18%. OCT imaging showed no plaque protrusion. Initial MLD of 0.74 mm increased to 2.3 mm.
There were no further adverse cardiac events after a mean follow-up of three months in four of the six cases. Patient number four suffered from in-stent restenosis after three months and this was treated with implantation of DES (TAXUS). Patient number five, with serious under-expansion due to multiple overlapping stent struts, suffered again from in-segment restenosis after two months, this time treated with a DES (XienceV).
Discussion
Covered stents using polytetrafluoroethylene (PTFE) membranes were designed to entrap friable degenerated plaque against the graft wall. But the promising preliminary observations10 were not confirmed by large randomised trials11,12. The SYMBIOT III randomised multicentre trial compared a self-expanding PTFE-covered stent with commercially available bare metal stents (BMS) in 400 SVG patients. There was no difference in the incidence of MACE between both groups (30.6% Symbiot, 26.6% BMS, P=0.43)13.
Two covered stents were extensively studied in SVGs. The PTFE-covered stent required very aggressive dilatation pressures (>18 atmospheres) for expansion because the PTFE membrane was thick and sandwiched between two stents. The self-expanding SYMBIOT stent had poor control of positioning due to the cumbersome deployment mechanism which favoured upward displacement of friable plaque during expansion, a possible cause of the frequent proximal edge restenosis.
Because of the negative results of all the randomised studies (RECOVERS, STING, SYMBIOT III), covered stents were withdrawn or their use was limited to the treatment of aneurysms or acute vessel rupture.
These preliminary observations indicate that the PCS are applicable in degenerated SVG lesions. The low pressure expansion required to prevent membrane damage and the uneven initial expansion limited to the edges are beneficial features in the treatment of degenerated SVG lesions, preventing squeezing of debris downstream.
Unlike for most native coronary lesions, there is still no convincing proof of better long term outcome with DES in SVG PCI14-16. In the randomised DELAYED RRISC trial with 75 patients undergoing SVG PCI using sirolimus eluting stents (SES) or BMS, the rates of target vessel revascularisation (TVR) at 32 months were not significantly different: 34% for SES vs. 38% after BMS (P=0.74). Periprocedural MIs were more frequent (18 vs. 5%, NS) and mortality significantly higher in the SES group (29 % vs. 0 %, P<0.001), with one death caused by definite very late stent thrombosis and three sudden deaths, possibly because of the higher MI rate17.
Direct stenting is also possibly beneficial to trap plaques at risks18, but in our series it was possible only in four lesions, which were not very severe. PCSs have a high profile compared to conventional stents and the difficulties in delivery observed in the third case call for further miniaturisation of the device.
Postdilatation may also cause plaque debris release, but we did not observe flow impairment after postdilatation of PCS. In a small study comparing PTFE-covered stents vs. BMS, Blackman et al reported that in the PTFE group, distal embolisation occurred only in two cases after initial stent implantation; in the remaining seven patients it was seen only after postdilatation (P=0.05)19, possibly due to squeezing of the plaque from the covered stent. The pockets of elastic pericardium protruding into the lumen (Figure 1d) may represent a reservoir for storage of the incompressible, friable, necrotic plaque. This sequestration of debris in multiple pericardium cells is very different from the situation observed with PTFE covered stents, acting as cylinders lying on the degenerated plaque. Long-term follow-up of the PCS is required to determine whether the more biocompatible equine pericardium also serves as a possible barrier to smooth muscle cell migration, thus reducing restenosis rates.
OCT was instrumental, both in vivo and in vitro, for elucidating the stent mechanism. OCT was also practically helpful in guiding the procedure, assessing the diameter of the vessel (an essential measurement to select a device with a limited range of expansion), the length of the degenerative lesions, complete apposition and presence of residual plaque protrusion. To our knowledge this is the first report of an OCT study in SVGs, because the traditional method of blood displacement using balloon occlusion was not suitable for use in large vein grafts. The thin OCT imaging wire advanced via an OTW microcatheter has a low risk of plaque displacement and offers excellent visualisation of superficial plaques. OCT ruled out thrombi as the cause of the angiographic intraluminal defects, a diagnosis otherwise impossible with angiography or IVUS.
Limitations
The optimal duration of double antiplatelet therapy after PCS implantation is unknown.
There is also the possibility that the high-profile PCS (0.0669 in.) may lead to significant distal embolisation when crossing the target lesion5. Therefore, PCS should be considered not as an alternative to EPD, but an additional device to reduce massive embolisation overloading EPD and to prevent late dislodgement of friable plaques in the first hours after stenting, for which EPDs are of no value, as shown in carotid stenting by the development of strokes and TIAs minutes or hours after deployment.
Currently the largest available PCS is 4.0 mm in diameter, expandable to a maximum of 4.56 mm without risk of membrane rupture. In many venous grafts this may be insufficient. Our aggressive deployment pressure in case one may have damaged the pericardium, which has a limited distensibility and led to the plaque protrusion treated with an additional PCS
Conclusions
These cases of OCT guided PCS for degenerated SVG lesion offer insight into the mechanism of protection against distal embolisation, elucidated by the appearance of these stents after deployment in vivo and in vitro.

