Introduction
Despite procedural refinements, coronary artery bypass graft (CABG) patients experience a predictable graft failure rate. Approximately one-third of the patients requiring a CABG procedure will need a second or even a third operation1, 10 years after their operations, 50% of these grafts will close2.
Since medical therapy alone is limited for patients with occluded or significantly obstructed grafts, percutaneous coronary intervention (PCI) has received interest as an alternative treatment option. PCI in grafted venous segments, as opposed to native coronary arteries, is subject to a particular set of limitations. Due to the fibrotic nature of the mid-graft lesions, and the high compliance of the graft wall, PCI may succeed only in radially stretching the vein graft during balloon dilatation, returning the lumen to its original (pre-dilatation) shape. The use of stents in the saphenous vein graft (SVG) intervention is also associated with a higher rate of procedural complications (i.e., distal embolisation, slow flow, CPK rise) compared to native coronary arteries3,4.
In addition to the difficulties of treating SVG lesions, a high likelihood of distal embolisation is associated with significant mortality and morbidity. There is also a high restenosis rate of 36% in non-occlusive lesions and up to 50% in occluded grafts. These difficulties are largely related to the extent, severity and composition of the atherosclerotic plaque in the SVG, which progresses unrelentingly over time. Ten years after bypass operation almost 50%-60% of the vein grafts are occluded5.
This particular plaque material may have a tendency to protrude through the stent struts and prolapse into the lumen, making this area prone to recurrence. The use of stents have improved the results of percutaneous revascularisation of obstructed vein grafts, but they have not demonstrated any reduction in the elevated restenosis rates. In addition, the long-term clinical event rate is still high compared with intervention in native vessels6.
Recent evidence confirms the complicated treatment of SVGs with bare metal stents (BMS) showing a target vessel revascularisation (TVR) rate of 37%7. Other studies, comparing BMS and drug eluting stents (DES) show no difference in the long-term outcomes of PCI on SVG, with a MACE of 46% and 50% at 33 months for BMS and DES respectively8 (MACE were defined as a composite of cardiac death, MI, and clinically driven TLR). Therefore, it will be desirable to have a mechanical barrier to prevent this event. Membrane covered graft stents have been designed to locally trap friable plaque against the graft wall and reduce the degree of subsequent neointimal proliferation inside the stent6.
Synthetic material has been used to cover stents with different modalities. Earlier data suggested that stents covered with a PTFE membrane might be associated with a low complication and restenosis rate in venous bypass grafts. However different trials have failed to demonstrate a superiority of the PTFE-membrane-covered stent graft compared with a conventional stent with respect to acute results, restenosis, or clinical event rates9,10.
As a result, alternative methods, basically embolic protection devices (EPD) have been developed in order to complement the treatment of SVG providing a safeguard to prevent the friable material from distally embolising in the SVG/coronary anatomy. However, some claim that SVGs may continue to shed some debris, even after the PCI has been completed and the EPD is no longer in place11.
As PTFE has not been effective in addressing the SVG problem, the need for a different material to graft the stents exists. Lately, a new device has begun being used in the European market, the pericardium covered stent, which may provide favourable results in the treatment of this difficult subset of patients.
Device description
The pericardium covered stent (PCS) is a percutaneous implantable device consisting of an equine pericardium cylinder covering a stainless steel stent pre-mounted on a delivery catheter and stored in a specially designed package to provide the device with a two year shelf life. The PCS is 6 Fr compatible for sizes 3.0 and 3.5 mm and a 7 Fr compatible for 4.0 mm diameter.
Currently available are two CE certified brands: Over and Under® and AneuGraft® which are specially designed for use in tortuous vessels. The devices are manufactured by ITGI Medical (Or Akiva, Israel).
Pericardium as a graft material
Pericardium tissue has been used extensively as a bio-material in surgical procedures ranging from heart valve leaflet repair, to hernia repair for more than 30 years. Several studies confirm the benefits of the pericardium when compared to other grafting materials. Pericardium has demonstrated a statistically significant decrease in intraoperative suture line bleeding when compared with Dacron12. It shows a better performance in regards to adhesion formation, wound maturation, bursting pressure, and tensile strength when evaluated in fascia lata abdominal wall repair13. Out of eight materials evaluated for pericardial substitutes for primary closure, the results suggested that pericardium tissue was an excellent substitute; the plastic materials were less impressive in that they caused epicardial reactions and showed signs of structural deterioration14.
A comparison of glutaraldehyde treated bovine and equine pericardium, formaldehyde treated bovine pericardium and PTFE as pericardial patches in dogs showed that the patch type did not significantly alter the patch behaviour. Over the years, it has been shown that glutaraldehyde cross linked pericardium tissue is an excellent surgical mesh/matrix material that can be used for cardiovascular and general surgical procedures, it is then thought that a pericardial cover might be a suitable alternative for covered stents.
Angele et al16 studied the influence of different species on physio-chemical properties of crosslinked collagen. Specifically, they looked at the resistance against collagenase digestion, swelling ratio, amino acid sequence, shrinkage temperature, ultrastructural matrix morphology, crosslinking density and stress-strain characteristics. Based on the results they concluded that the ultrastructure, the crosslinking density and the strain at rupture between collagen matrices of both species showed no significant differences. For tissue engineering purposes, the higher enzymatic stability, the higher form stability, as well as the lower risk of transmissible disease made the case for considering equine-based collagen.
Initially the stent was developed based on bovine pericardium, further events as the spread of BSE and the literature search conducted, justified a change of material to equine pericardium which is still currently used.
Stent design
The stent is manufactured out of a 316L stainless steel laser slotted hypo-tube with multiple segments, which provides a more predictable stent expansion and allows for the pericardial cylinder integration. The design provides high flexibility with excellent radial force. An equine pericardium cylinder with a 105µ thickness is manufactured by a longitudinal suture line of non-absorbable suture. The cylinders are manufactured in a special way that allows for post implantation over-expansion, however care should be taken not to expand the stent beyond the manufacturer recommended diameter. The equine pericardium cylinder is positioned under the proximal and distal elements of the stent and over its main body. This design minimised the amount of steel in touch with the intimal layer of the blood vessel. The proximal and distal elements have opposed bars at each end that will be under the cylinder. The cylinder is then fixed to the stent by non-absorbable sutured knots.
This configuration allows for the single stent to be single layer covered, avoiding folding or double layers that could increase the profile, compromise the trackability and the flexibility of the device. (Figure 1)

Figure 1. The pericardium covered stent.
The deliverability of the pericardial covered stent was compared with PTFE covered stent (Jostent, Abbott Vascular, Redwood City, CA, USA) in phantom and porcine coronaries. The pericardial covered stent appeared superior in deliverability in both model systems, going further deep in phantoms by 12±10 mm and in porcine vessels by 19±13 mm (p<0.05 in both models)17.
Balloon catheter
The stent assembly is mounted on a special balloon catheter designed to initiate its expansion at the edges so that any friable material within the SVG would be trapped between the pericardial layer and the graft wall (Figure 2b).
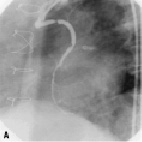
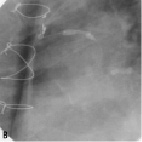
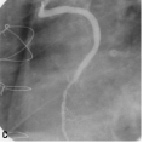
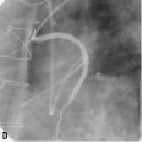
Figure 2. A sample procedure on a nine year old SVG (images courtesy of Dr. Jorge Gaspar, Mexico). a) Lesion pre-treatment. b) The pericardium covered stent deploys its edges first to trap the plaque. c) Final acute results. d) Results of the nine months follow-up.
The balloon catheter delivery system is a semi-compliant rapid exchange design, 0.014” guidewire compatible with a stainless steel hypotube and a transition zone.
Stent packaging
The pericardium covered stent assembly is liquid chemical and ethylene oxide sterilised. It is packaged in a tubular container, a “wet container”, containing sterile glutaraldehyde solution and then placed on a plastic tray suitable for EO sterilisation.
Indications for use
The pericardium covered stent is intended for intraluminal chronic placement after PTCA procedures that have been performed in coronary arteries, aorto-coronary bypass grafts, or suitable size vessels. It may be used for the following indications: coronary bypass-vein graft stenosis, coronary bypass-vein graft aneurysm. In emergency situations the device also can be used for: acute coronary artery perforation, acute coronary artery rupture, and coronary artery aneurysm. However, clinical studies have not been conducted for these emergency situations.
Figure 2 shows a sample procedure in a nine year old SVG indicating the deployment and follow-up.
Tips & tricks for delivery
Rinse procedure
Prior to deployment, in a sterile field, the stent has to be rinsed in physiological saline for two minutes. Stent assembly should be retrieved from the ‘wet container’ and submerged in saline to remove the preservative solution. If not used immediately, the stent should be kept wet at all time.
Guidewire and guiding catheter
Pericardium covered stents are available in 3.0, 3.5 and 4.0 mm in diameter and 13, 18, 23, 27 mm in length. Stent Ø 3.0-3.5 mm is 6 Fr guiding catheter compatible, with a minimum I.D. 0.070”. The PCS Ø 4.0 mm is 7 Fr guiding catheter compatible with a minimum I.D. 0.078”. The PCS should be used with 0.014” guidewire and large lumen “Y” connector (i.e., 0.125”). It is recommended not to use a “push button” like haemostatic valves.
Pre-dilatation
The pericardium covered stent is not intended for direct stenting. Pre-dilatation of the lesion is required with an appropriate size balloon prior to using the PCS.
Stent deployment and over-expansion
Stent nominal deployment pressure is 5 atm. Over-expansion may be performed according to maximum allowed pressure as indicated on the compliance chart.
Clinical experience
SLEEVE I
SLEEVE I was an open-label, non-randomised registry study of 15 eligible patients at two centres who required interventional treatment in saphenous vein grafts (SVG). This was designed in order to evaluate the safety and feasibility of the pericardium covered stent for the treatment of saphenous vein graft stenosis. The primary safety objective of this study was to evaluate the types and frequencies of clinical, angiographic, and other complications.
All patients met the inclusion/exclusion criteria specified in the protocol. The trial enrolled patients who had symptoms suggesting a de novo and/or restenotic lesion(s) in a saphenous vein graft. Each patient was treated with the bovine pericardium covered stent. Baseline demographics and clinical characteristics revealed a mean age of 66±10 years, with 87% (13/15) males and 47% (7/15) with a history of diabetes mellitus. For each patient included in the SLEEVE trial, a baseline angiogram was performed and analysed by an independent angiographic core laboratory (Cardiovascular Research Foundation, New York, NY, USA).
The results from this study of 15 patients, with 22 lesions, was a procedural success rate of 100% (defined as attainment of a <50% residual diameter stenosis using any percutaneous method), no in-hospital major adverse cardiac events (MACE) defined as cardiac death, myocardial infarction (Q-wave and non-Q-wave), and the need for repeat intervention of the previously treated lesion with any percutaneous technique or bypass surgery and a clinical success rate of 93.3% (defined as successful delivery of the study device with attainment of a less than 50% residual stenosis, and without the occurrence of any major adverse cardiac event at 30 days from the procedure). Diameter stenosis improved from 65.63±16.56 to 18.56±8.94 in all 22 lesions after study stent deployment (p<0.001). The technical success, being the ability to reach the lesion, accurately position, and successfully deploy the pericardium covered stent was 100% with no device failures. At 30-day follow-up, one patient had expired due to cardiac causes, 10 days after enrolment in the study. The event was adjudicated by the Clinical Events Committee as related to the procedure, and the MACE at 30 days was 6.7% (1/15).
SLEEVE II
SLEEVE II was a multicentre, multi-national registry study of 47 eligible patients, requiring interventional treatment in saphenous vein grafts (SVG) that met study inclusion/exclusion criteria. The study involved the collection of demographic, clinical, procedural and angiographic data on the targeted patient population, including in-hospital and 30-day follow-up data.
The purpose was to establish the clinical safety and efficacy of the pericardium covered stent in diseased saphenous vein grafts. The primary endpoint of this study was to evaluate MACE at 30 days, defined as cardiac death, myocardial infarction (Q-wave and non-Q-wave), and the need for repeat intervention of the previously treated lesion with any percutaneous technique or bypass surgery.
The trial enrolled 47 consecutive patients at six sites, with previous saphenous vein graft surgery, who had symptoms suggesting a de novo and/or restenotic lesion(s) in the SVG (total 58 lesions). Baseline demographics and clinical characteristics revealed a complicated typical SVG patient population with a mean age of 67.83±9.12 years, with 83.0% (39/47) males and 46.8% (22/46) with a history of diabetes mellitus. Detailed demographics are shown in Table 1.
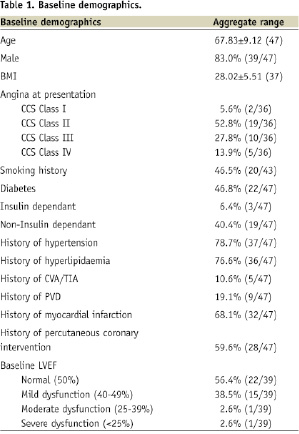
All patients had baseline angiography and 46 patients had their baseline films analysed independently by the angiographic core laboratory at the Cardiovascular Research Foundation, New York, NY, USA. All patients with successfully treated target lesions were followed for 30 days. Table 2 summarises the angiographic findings pre- and post- treatment.
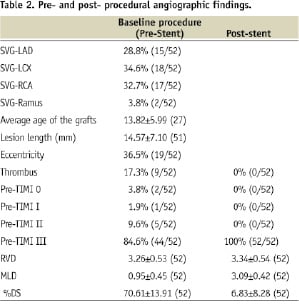
Results
The total cumulative MACE incidence at 30 days was 10.6% (5/47) shown in Tables 3 and 4, and are as follows: one patient died due to pulmonary oedema not related to the device.
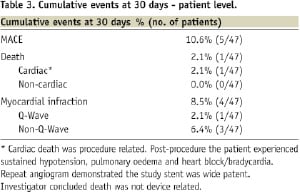
At day one after enrolment in the study, three patients experienced post-procedure non-Q MI (all were procedure-related; however, two of them were database triggered). One patient experienced post-procedure acute QWMI (not related to the device). All events were adjudicated by an independent CEC from the Cardiovascular Research Foundation.
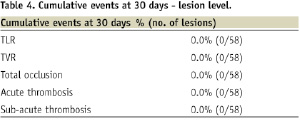
No patient underwent revascularisations within the 30-day interval. All sites reported MACE, device failures, and cardiac triggers that met the myocardial infarction criteria from the database and were adjudicated by an independent Clinical Events Committee (CEC). No acute or sub-acute stent thrombosis was reported.
Technical success was defined as the ability to reach the lesion and accurately position and deploy the pericardium covered stent and was seen to be 85.9%. Procedural success, defined as residual diameter stenosis (visually and/or QCA estimated) <50% in the lesions treated with the study stent, without any in-hospital MACE was 89.1%.
The study conclusions stated by the CRF report was that:
“In patients undergoing percutaneous coronary interventions for single or multiple lesions in saphenous vein graft vessels, the use of bovine pericardium covered stent in the 47 patients enrolled was associated with 10.6% (5/47) cumulative Major Cardiac Adverse Events (MACE) at 30 days. The results prove that the bovine pericardium covered stent is safe to be used in patients with de novo or restenosed saphenous vein grafts lesions.”
Other clinical works have been published with the pericardium covered stent in regards to aneurysm and HOCM treatment18,19 with successful results as well.
Conclusions
A literature review has revealed the biocompatibility of the pericardial tissue as a graft material. Also, a further search demonstrated no commercially available synthetic grafts smaller than 6 mm in diameter, the use of ePTFE covered stents has resulted in negative results in SVG and native coronary arteries. Although short term information is available, the pericardium covered stent did not show any signs of acute or sub-acute thrombosis and presented comparable clinical results to alternative options in the market, providing the additional benefit of treating situations where a bare metal or drug eluting stents cannot perform. Such cases would be of friable material angioplasty, especially in SVG or where thrombus is present, exclusion of aneurysms of the circulatory system and resolving life threatening perforations. The design of the pericardium covered stent allows for improved trackability17 and performance compared to other covered stents available in the market. As the product is being introduced into the “real world”, more information will become available allowing us to better understand the benefits provided by the device and plan for wider indications for use.
The current clinical results, mainly those of the SLEEVE II trial and early experience presented in the published literature, suggests that using a pericardium covered stent is justified in clinical practice, outweighing the possible risks to treat the difficult and complicated SVG, as well as the life threatening complications from aneurysm and perforations.
Acknowledgements
We would like to thank Danna Moran for her contribution to this article.
Online data supplement
Video 1: lesion pre treatment
Video 2: Pericardium Covered Stent deployed
Video 3: final acute results
Video 4: 9 months follow-up

