Abstract
Saphenous veins remain a source of conduit for use in surgical coronary bypass graft revascularisation procedures. Saphenous vein grafts have a progressive closure rate estimated to be 12% to 20% at the end of the first year, and approximately 50% by 10 years. Regarding secondary revascularisation in these cases, reoperation carries substantially increased morbidity and mortality rates, making saphenous coronary intervention, in particular stent implantation, a more attractive means of revascularisation. However, this procedure carries a significant risk of major adverse clinical events, predominantly myocardial infarction or reduced antegrade flow (non-reflow phenomenon), mainly due to distal embolisation of atherothrombotic debris and distal microvascular occlusion. Embolic protection devices are used to reduce the risk of distal embolisation. There are two different designs: filter and occlusion-aspiration devices. In this article we present the different systems of embolic protection devices in saphenous percutaneous intervention and the previously published information is reviewed.
Abbreviations
SVG: Saphenous vein graft
PCI: Percutaneous coronary intervention
MACE: Major adverse cardiac event
MI: Myocardial infarction
EPD: Embolic protection device
The scope of the problem
Saphenous veins remain a source of conduit for use in surgical coronary bypass graft revascularisation procedures. Saphenous vein grafts (SVG) have a progressive closure rate, estimated to be 12% to 20% at the end of the first year and approximately 50% by 10 years1. Graft disease results from intimal hyperplasia, mostly within the first year, followed by atherosclerotic plaque build up with superimposed thrombus2. Some characteristics differentiate this lesion from that of the native vessel: lesions are diffuse and concentric with thin or absent fibrous cap; foam cells and lipid debris are in contact with blood; lesions are friable and affect large vessels. Regarding secondary revascularisation in these cases, reoperation carries substantially increased morbidity and mortality rates3, making SVG percutaneous coronary intervention (PCI), in particular stent implantation, a more attractive means of revascularisation4,5. Percutaneous interventions in SVG represent 10-15% of the totality of PCI in United States6.
However, SVG PCI carries a significant risk of a major adverse clinical events (MACE), predominantly myocardial infarction (MI) or reduced antegrade flow (non-reflow phenomenon)7, although mortality may be increased by 10-fold compared with PCI in native vessels8. The major factor is distal embolisation of atherothrombotic debris and distal microvascular occlusion7,9-14. Embolic protection devices (EPD) are used to reduce the risk of distal embolisation during SVG PCI. In the present article, we present the different systems of embolic protection devices in saphenous percutaneous intervention and the previous published information is reviewed.
Type of embolic protection devices
There are two types of EPD: filter and occlusion-aspiration devices. These systems have different characteristics and to date none has demonstrated enough advantages over the other mechanisms to be ideally recommended universally.
Distal embolic filter devices maintain distal perfusion and allow injection of contrast medium during PCI while trapping most particulate debris. Embolic filters include the FilterWire-EZ™ (Boston Scientific Inc, Natick, MA, USA), the Interceptor Plus Coronary Filter System™ (Medtronic Vascular, Santa Rosa, CA, USA) and the Spider™ (eV3, Inc., Plymouth, MN, USA) (Figure 1). The advantages are that they preserve antegrade flow, contrast imaging is possible throughout the procedure and they are very simple to use. However, they are associated with limitations such as the fact that may not be able to capture all the debris, it may also be difficult to evaluate retrieval of the debris during the procedure, delivery catheters may cause embolisation before filter deployment and the possibility of snagging of the retrieval sheath on the stent.
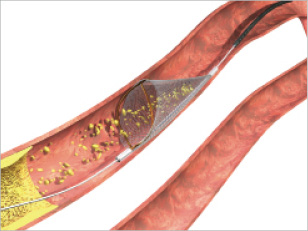
Figure 1. Picture of the Spider™.
Based on a different concept, proximal and distal occlusion-aspiration devices suspend antegrade flow during the intervention. The stagnant column of blood containing particulate debris and humoural mediators is aspirated before relief of occlusion and restoration of flow. The group of proximal occlusion devices (Figure 2) is represented by the Proxis Embolic Protection System™ (St. Jude Medical, Minneapolis, MN, USA). The advantages of this device are the myocardial protection during wire crossing, no landing zone is required, the 0.014” wire can be chosen, the utilisation of large lumen catheters allows aspiration of large thrombus and finally the lesion can be visualised by contrast suspension. Unfortunately, they also have limitations: there is no antegrade flow with the subsequent possibility of myocardial ischaemia during the procedure, its utilisation is more complex than the distal filters and they can not be used in ostial disease.
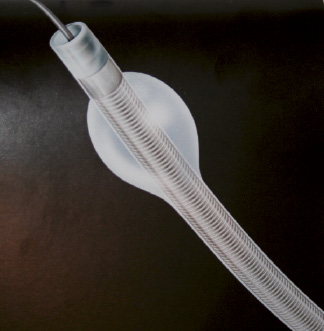
Figure 2. Picture of the Proxis embolic protection system.
Finally, the distal occlusion-aspiration devices are represented by the PercuSurge GuardWire™ (Medtronic Vascular, Santa Rosa, CA, USA) (Figure 3) and the TriActive System™ (Kensey Nash, Exton, PA, USA). It is easy not only to cross the lesion with the device but also to retrieve it at the end of procedure, they are able to aspirate large and small particles and they reliably trap the debris. The disadvantages are that there is no antegrade flow causing patient intolerance in 5-8% of cases, balloon-induced injury may occur if they are not used carefully and it is difficult to get adequate imaging during the procedure.
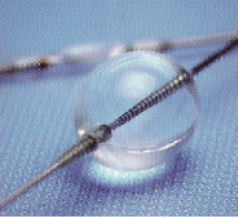
Figure 3. PercuSurge GuardWire system.
Although, as has been mentioned above, none of these devices has demonstrated more efficacy than others in a randomised trial, some recommendations can be made based on anatomical considerations and tolerance of absence of antegrade flow: distal occlusion may be the preferred choice in cases of proximal disease with high plaque or thrombus burden; filters may be proposed in cases of poor tolerance for ischaemia or single remaining grafts without distal disease and proximal occlusion devices would be indicated in grafts with distal disease, especially in relatively straight vessels.
Key evidence of embolic protection devices
Previous studies have been published evaluating the results of EPD. Interestingly only one randomised controlled trial, the SAFER (Saphenous vein graft Angioplasty Free of Emboli Randomised) trial, demonstrated a reduction in the endpoint with the EPD versus conventional guidewire use. Subsequent EPDs have only been tested against an active control group in noninferiority comparisons (Table 1 and Figure 4).
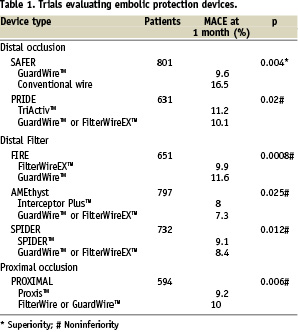
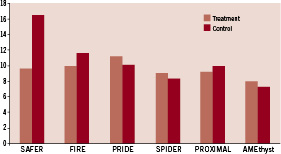
Figure 4. MACE at 30 days of different trials and registries. Only SAFER demonstrated a reduction in the endpoint with the EPD versus conventional guidewire use. Subsequent EPDs were tested against an active control group in noninferiority comparisons.
The SAFER trial15: in this study, 801 patients with SVG stenosis were randomly assigned to stent placement over the shaft of a distal protection device (PercuSurge GuardWire™) or a conventional angioplasty guidewire. The use of the EDP device during stenting, with or without the concomitant administration of a glycoprotein IIb/IIIa inhibitor, was associated with a highly significant reduction in MACE. The primary endpoint, a composite of death, myocardial infarction, emergency bypass, or target lesion revascularisation by 30 days, was observed in 65 patients (16.5%) assigned to the control group and 39 patients (9.6%) assigned to the EPD. This reduction was driven by myocardial infarction (8.6 vs. 14.7%) and non-reflow phenomenon (3 vs. 9%). In this study, use of a distal balloon occlusion device was independently predictive of lower 30-day rates of MACE. The risk of 30-day MACE is increased in more diffusely diseased grafts and in bulkier lesions, but a significant benefit of the GuardWire was seen across all levels of MACE risk rather than just those perceived to be at highest risk16.
In the FIRE (FIlterWire EX™ Randomised Evaluation) trial17, 651 patients undergoing percutaneous intervention of saphenous vein lesions were randomised, in a noninferiority study, to distal protection with the filter-based FilterWire EX versus the GuardWire balloon occlusion and aspiration system. Initial procedure success was excellent for both devices (97 and 96% respectively). The primary endpoint, the composite incidence of death, myocardial infarction or target vessel revascularisation at 30 days occurred in 9.9% of FilterWire EX patients and 11.6% of GuardWire patients. The outcome, in term of the composite endpoints, remained comparable at six months; but an additional 10 percent of patients had a new event (21.9 vs. 19.3% overall)18. Target vessel revascularisation was required in 9.1%.
The PRIDE (PRotectIon During saphenous vein graft intervention to prevent distal Embolisation) study19 is a prospective randomised non-inferiority trial comparing outcomes of 631 patients with coronary ischaemia and lesions in SVG treated with TriActiv System, a balloon-protection flush and extraction device, versus a control group treated with GuardWire system or Filterwire EX. In terms of the primary endpoint, composite incidence of cardiac death, myocardial infarction, or target lesion revascularisation at 30 days, the TriActiv System was not inferior to the other devices (11,2 vs. 10,1%) but patients in the TriActive group were more like to require blood transfusion. This was associated with technical problems in the design of the haemostatic valve in combination with the use of 8 Fr guiding catheters.
The PROXIMAL (PROXIMAL protection during saphenous vein graft intervention) trial20 prospectively randomised a total of 594 patients undergoing stenting of 639 saphenous vein grafts using a noninferiority design to compare two treatment strategies: control (distal protection whenever possible, with no protection when lesions where not amenable to distal protection) or test (proximal protection when possible, distal when not). In both groups, when distal protection was used, either the FilterWire or the GuardWire could be used. The Proxis Embolic Protection System is a single operator catheter that is deployed proximal to the target lesion before crossing. It is designed with the aim of circumvent the limitations of distal embolic protection. The primary endpoint was a 30-day composite of death, myocardial infarction and emergent coronary artery bypass graft surgery or target vessel revascularisation. When analysed by intention to treat, proximal protection was not inferior. The primary endpoint occurred in 10% of control patients and 9.2% of test patients. When analysed by device used, the composite endpoint was less frequent in the proximal protection (7.1% vs.11.7%). When lesion where amenable for either device, the primary end point occurred in 7.4% of proximal protection patients and 12.2% of distal.
The AMEthyst (Assessment of the Medtronic avE inTerceptor sapHenous Vein graft filter sYSTem)21 is a randomised, noninferiority trial that enrolled 797 patients undergoing PCI with stenting of SVG stenosis. Patients were assigned to either the Interceptor (a steerable, expandable vascular filter) or control distal protection (GuardWire or FilterWire EZ). Primary endpoint (composite occurrence of death, myocardial infarction or urgent repeat revascularisation through 30 days) was observed in 8% and 7.3% of Interceptor and control-treated patients respectively, indicating that this device is not inferior when compared with the control.
Finally, the SPIDER (SaPhenous vein graft In a Distal Embolic protection Randomised) trial22 is a randomised, noninferiority trial comparing the Spider Filter System with the FilterWire or Guardwire. In this trial again there were no differences between the two arms of treatment (9,1% vs 8,4%).
In an attempt to characterise the level of baseline risk and the possible existence of predictors of MACE in SVG PCI, 3,992 patients with 4,314 lesions, enrolled in five randomised trials and one registry, were analysed23. The trials pooled for analysis were: SAFER, FIRE, CAPTIVE24, PROXIMAL, SPIDER and BLAZE II25. When 30-day MACE predictors were identified, the strongest independent were SVG degeneration score (P<0.0001) and estimated plaque volume (P<0.0001). Angiographic evidence of thrombus (p=0.003), increasing patient age (p=0.005), glycoprotein IIb/IIIa inhibitor use (p=0.02) and current smoking (P=0.03) were also independent predictors of adverse outcome. A very important conclusion of this study is that although the absolute risk for MACE increases with higher SVG degeneration score and plaque volume, a relative benefit is maintained across all categories of risk.
Other issues
A very important matter is that, despite the demonstrated benefit and the current recommendation IB in the ACC/AHA and ESC guidelines26,27, the utilisation of these devices is still far from the desired rates. In an analysis of 19,546 SVG PCI procedures in the American College of Cardiology-National Cardiovascular Data Registry from January 2004 to March 2006, EPD were only used in 22%. Nineteen percent of these centres did not use EPDs and 41% used them in <10% of cases. Variables associated with their use were higher age, male gender, older grafts, longer lesions and class C lesions. Patients were less likely to receive an EPD if they had class <3 TIMI flow or previously treated lesions. There was a weak correlation between annual hospital PCI volume and EPD use. In this analysis, EPD use was independently associated with a lower incidence of no-reflow but not in-hospital mortality28. In the same way, in the Spanish registry of 200729, the protection devices were used in only 19%.
Various reasons may be suggested to explain the clear disparity between the current type I recommendation and real world practice. Firstly, SVG interventions represent a low percentage of the total amount of PCI, specially outside the United States. Although, in the US, the percentage may reach 10-15%6, in European countries it is far less common, as is demonstrated in the mentioned Spanish registry, where the SVGs were only 2,6%29. This fact could mean that the actual number of percutaneous interventions in SVG per operator per year may be too low to get the necessary skills with these devices. Secondly, the complex properties of some of these devices may also play a role. Lastly, the main reason may be the current evidence available: as was mentioned earlier, only the SAFER trial compared one device with conventional wire and the rest of the studies were noninferiority designs compared with the GuardWire device. In SAFER15, although the primary endpoint was achieved, it was a composite endpoint of death, MI and non-reflow, and there were no differences in death at 30 days. This fact has been confirmed in the above mentioned American College of Cardiology-National Cardiovascular Data Registry and this lack of benefit in the hard endpoint of in-hospital death may have forced a limitation in their use. Probably this reason is the main one, because in that registry there was a weak correlation between annual hospital PCI volume and EPD use.
Clinical implications
It has been clearly proven that PCI in SVG is associated with a higher incidence of myocardial infarction and death7,8. Unfortunately, although IIb-IIIa inhibitors are a valuable tool in the reduction of cardiac events in native vessels, their benefit in SVG has not been demonstrated30. What this means is that precisely in one of the most unfavourable scenarios, the usefulness of these valuable drugs is lacking. Frequently, the graft has degenerated, and all efforts to minimise embolisation and non-reflow must be attempted. Direct stenting has demonstrated a reduction in CKMB and troponin release in this context31-33, so it should be the preferred approach in all the possible cases. Besides, EPD should also be used in as many cases as possible. Their benefit in terms of MI and non-reflow have been proven, and the absence of benefit in death in the SAFER trial and in the American College of Cardiology-National Cardiovascular Data Registry should not be a reason for under-use. We should remember that in this last and larger cohort, only the in-hospital deaths were registered, while in the SAFER trial it was the 30-days mortality. It has been previously reported that there is a linear relationship between CK-MB and troponin release and death in follow-up34,35.
Final remarks
In summary, saphenous vein graft percutaneous intervention is associated with a high risk of MACE, mainly periprocedural MI or reduced antegrade flow, but also increased mortality. The major factor is distal embolisation of atherothrombotic debris and distal microvascular occlusion. The use of embolic protection devices shows a benefit in reducing major adverse cardiac events and that effect is maintained across all categories of risk. However, despite the demonstrated advantages associated with these devices, their utilisation is much lower than the desired percentages. Due to this, and given the lack of benefit of IIb-IIIa inhibitors in this complicate setting, special effort should be done in trying direct stenting and the utilisation of embolic protection devices in all the possible cases of percutaneous interventions in saphenous vein grafts.

