Background
The percutaneous treatment of patients with obstructive atherosclerotic disease in saphenous vein bypass grafts (SVG) remains a challenge. Patients undergoing SVG intervention are often old and suffer significant comorbidities. Moreover SVG usually present a degenerated pattern of atherosclerosis, with complex, friable, thrombotic-prone lesions. SVG interventions carry a high risk of acute complications, mainly distal embolisation leading to periprocedural myocardial infarction (MI), and poor long-term outcomes, mainly restenosis.
Indications
Due to the increased risk of periprocedural MI related to SVG interventions, percutaneous coronary intervention (PCI) of the native grafted vessel if technically feasible is usually preferred over PCI of the degenerated SVG. However, when PCI of the native vessel is not possible, the clinical benefit of a high-risk SVG intervention should be balanced against the risk of morbidity and mortality of repeat coronary artery bypass surgery (CABG). The latest guidelines recommend PCI in the following circumstances:
– Early ischaemia (<30 days) after CABG, when technically feasible.
– Ischaemia after CABG in patients with discrete SVG lesions and preserved left ventricle (LV) function, mainly if the left internal mammary artery (IMA) is patent.
– PCI is not recommended in patients with total SVG occlusions, or multiple target lesions and impaired LV function, unless repeat CABG poses excessive risk.
– CABG is usually reserved for patients who cannot have adequate percutaneous revascularisation or for those who may gain an additional benefit from CABG, such as those with previously unused left IMA.
In summary, PCI is preferred over repeat CABG for early recurrent symptoms after CABG. However, most symptomatic post-CABG patients present with extensive native and graft disease where the revascularisation strategy must be based on careful risk/benefit assessment.
Difficulties and methods
Choosing the correct guiding catheter is the first important step. For the vein graft to the right coronary artery, originating most of the time from the right anterior surface of the aorta, the multipurpose catheter has the best alignment and support when performing the procedure either via the femoral or the radial artery. Engagement of the catheter can be performed in the left anterior oblique view. For vein grafts originating from the left anterior surface (usually to the left anterior descending/diagonal or marginal/circumflex arteries), Judkins right or left coronary bypass catheters are usually the best fitting in case of femoral access. If additional support is needed, Amplatz left catheters can be used. Engagement is feasible in the right anterior oblique view. When approaching these bypasses via the radial approach, we recommend avoiding the right radial artery, instead choosing the left radial artery. Guiding catheters of choice can be extra-back up shaped, multipurpose with a long distal arm, or Amplatz left catheters. Sometimes vein grafts to the circumflex originate from the posterior surface of the aorta. In this case, Amplatz left or multipurpose guiding catheters are preferred, either via femoral or radial approach. Also in this case engagement is performed in the right anterior oblique view.
Embolic protection devices (EPD: proximal or distal occlusion and aspiration devices or distal filters, Figure 1) have demonstrated value in decreasing the risk of embolisation and post-procedural myocardial enzyme elevation after SVG intervention, thus they should be advocated in any SVG procedure, however, due to economic reasons, they can eventually be avoided in cases of very focal lesions in small (<3.5 mm) grafts. In this case, a soft-tip coronary guidewire and direct stenting without pre- and post-dilatation should be the recommended strategy. Choosing the type of EPD depends mainly on the location of the lesion and on the operator’s experience. In case of lesions in the mid-SVG, any EPD is usable. For proximal/ostial lesions, distal EPD are recommended. For distal lesions, proximal EPD are chosen. The theoretical background for these choices relies on the fact that every EPD needs a vein graft disease-free landing zone of around 3-5 cm, proximally to the lesion for proximal EPD and distal to the lesion for distal EPD.
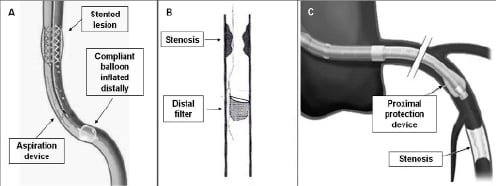
Figure 1. A. Distal occlusion and aspiration device. B. Distal filter. C. Proximal occlusion device.
When using proximal or distal occlusion EPD, the effectiveness of complete vessel occlusion should be assured. This means that a column of stagnant blood (checked with contrast) should be evident once the device is in place. If a filter is used, appropriate deployment of the filter (good apposition to the vessel wall) should be verified in two orthogonal views.
While stenting has been definitely proven superior to balloon only angioplasty for the treatment of SVG lesions, the choice of the type of stent (bare metal stent versus drug-eluting stent) is still a matter of debate. Drug-eluting stents are potentially a promise for the successful sealing of SVG disease, however available long-term safety and effectiveness data are conflicting and give reason for caution. In any case, direct stenting should be always attempted if deemed feasible, and in case the stent remains significantly under-deployed, high-pressure post-dilatation must be done with an EPD in place.
Once the stenting procedure is finished, care should be given to the collection of debris from the protection device. With proximal device placement, direct aspiration of at least 5 cc of blood from the device should be performed before releasing the occlusion. In case of distal occlusion device use, the specific manual aspiration device should be used, and two syringes of 20 cc of blood should be aspirated. If a filter is used, careful and complete closure of the filter is necessary before retrieval. We recommend performing the complete procedure under fluoroscopy. Final angiographic control of the stenting procedure without a device in place is mandatory at least in two orthogonal views.
Conflict of interest statement
The authors have no conflict of interest to declare.

