ABSTRACT
Aims: Percutaneous coronary intervention (PCI) in the total occlusion of aged saphenous vein grafts (SVGO) is difficult because of inadequate dilatation and distal embolisation. The aim of this study is to report the early experience and clinical outcome of SVGO PCI with a multistaged procedure.
Methods and results: Between January 2003-2006, we treated 20 consecutive patients (pts) admitted with the diagnosis of non-ST segment elevation acute coronary syndromes with culprit lesion located in SVGO. All of them underwent a three stage procedure.
Stage 1: recanalisation of graft with undersized balloon; Stage 2: anticoagulation therapy; Stage 3: treatment according to the angiographic result. We obtained vessel recanalisation with TIMI flow 1-2 in 14 pts (70%) by undersized balloon (mean diameter of 1,85±0,43 mm). The mean anticoagulation time was 11±7 days (range 1-20 days). At the Stage 3 all 14 SVG (100%) were patent. A focal lesion was present in 12 pts (86%) treated by stent implantation. In the other two pts (14%) the SVG had no residual lesion. The in-hospital major adverse cardiac event rate was 10%. No death, in-hospital late closure or major bleeding complication occurred. At 2-year follow-up one target vessel revascularisation and one in stent restenosis occurred.
Conclusions: The treatment of SVGO with staged procedure may be used to decrease thrombus burden prior to definitive “short” stent placement and improve the acute and long-term results.
Introduction
Compared to atherothrombosis in native coronary artery, plaques in saphenous vein grafts (SVG) are generally much larger, contain more lipids, foam cells, necrotic debris and, particularly in older degenerated grafts, there is often an overlying thrombotic material. These characteristics make the plaques much more friable and vulnerable, which explains the exceptionally high risk of distal embolisation.1 Even with the introduction of stents and distal protection devices the in-hospital and short-term outcomes of percutaneous coronary intervention (PCI) in SVG obstruction remains unsatisfactory.2 Percutaneous treatment of SVG obstructions is difficult because it is often associated with a poor procedural success and with high rates of restenosis and mortality at long-term follow-up.3 These poor results are partly due to procedural complications and partly due to the fact that symptomatic post coronary artery bypass graft patients have a higher incidence of cardiovascular risk factors, comorbidity and more extensive atherosclerotic disease. For these reasons, several authors suggested that totally occluded SVGs is “a challenge that should be resisted”.3 The aim of the present study is to report the early experience and the clinical outcomes of a new approach for percutaneous treatment of totally occluded SVGs based on a multi-staged procedure with implantation of stents after debulking of the thrombus.
Method
Population
The study population included twenty consecutive patients who had previous coronary artery bypass graft (CABG), admitted to our cardiology department between January 2003 and January 2006 with the diagnosis of acute coronary syndromes and in whom an occluded saphenous vein graft was present as a culprit lesion. The study population represents approximately 10% of all the percutaneous revascularisations performed on SVGs which in turn constitute 4% of the 4491 PCI performed in our institute over the same time period. Patients underwent coronary angiography and PCI because of unstable angina, according to Braunwald classification4, or non ST elevation myocardial infarction (NSTEMI)5. Patients with ST elevation acute myocardial infarction or previous PCI on the target vein graft were excluded. All patients provided written informed consent prior to the procedure.
Procedure and protocol
Detailed medical history, clinical examination, 12 leads ECG and trans-thoracic echocardiography were performed in order to estimate the duration of bypass graft occlusion, the ischaemic area and the culprit vessel. All patients received nitrates, beta blockers or Ca-Channel blockers (adjusted according to heart rate, blood pressure and recurrent ischaemia), aspirin (100 mg/day) and clopidogrel (loading dose 300 mg followed by 75 mg/day, maintained for six months after drug eluting stent implantation and one month after bare metal stent). Intravenous glycoprotein IIb/IIIa inhibitors were used administered upstream or during the PCI at the discretion of the operator.
Coronary angiography was done by femoral approach with a standard 6 Fr guiding catheter and analysed by two expert interventional cardiologists. Patients with TIMI flow 0 in a saphenous vein graft (proven to be the culprit lesion) and with native vessel untreatable by PCI, were included in the study population and the experimental approach consisted of the following three stages (Figure 1):
Stage 1 (Index procedure): Recanalisation of the totally occluded venous graft with undersized balloons to obtain antegrade flow (almost TIMI flow 1-2).
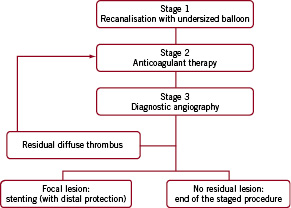
Figure 1. Staged procedure algorithm.
Stage 2: Treatment with glycoprotein IIb/IIIa inhibitors and anticoagulants. Patients received either Tirofiban -upstream use- in intensive care (0.4 μg/kg/min intravenously for 30 minutes followed by infusion of 0.10 μg/kg/min for 48-72 hours) or Abciximab in catheter laboratory (0.25 μg/kg intravenous bolus followed by infusion of 0.125 μg/kg/min for 12 hours). Unfractionated heparin (UFH) was started before the procedure and continued for 2-3 days after the index procedure (intravenous bolus 60 U/kg -maximum 5000 IU- followed by an infusion 12-15 U/kg/hour -maximum of 1000 U/h- adjusted after that to achieve one and half to two times the control value for activated partial-thromboplastin time). Low molecular weight heparin (Enoxaparin 100 U/kg twice per day) was used from day three up to Stage 3.
Stage 3: Optimisation of the treated SVG. A diagnostic angiography was performed within 15 days after the index procedure to detect: 1) the patency of the treated graft, 2) the presence of any residual lesion suitable for stenting. The presence of residual diffuse intragraft thrombus (several centimetres in length all along the vessel) was an indication to continue the anticoagulant therapy before further treatment. All procedures were performed using distal protection devices (SpiderFX™; ev3 Inc., Plymouth, MN, USA). Clinical follow-up was scheduled for all patients every six months up to 24 months by office visit or telephone interview. When the patient was not reachable, the information was sought from the referring physician, hospital electronic database or Municipal Civil Registries. Angiographic follow-up was performed only if clinically driven. This protocol was approved by the hospital ethic committee and is in accordance with the Declaration of Helsinki.
Definition
Technical success was defined as the ability to cross and open the occluded segment with no more than 40% residual stenosis in all views; procedural success was defined as a technical success and no major in hospital complications (death, need for emergency coronary revascularisation, myocardial infarction). TIMI flow grade was assessed as defined by TIMI study group.6 Major adverse cardiac events (MACE) were defined as all causes of death, myocardial infarction (MI) and target lesion revascularisation (TLR) or target vessel revascularisation (TVR) at follow-up. According to the universal definition of myocardial infarction7 we considered the spontaneous MI (Type 1) related to ischaemia due to primary coronary event; periprocedural MI Type 4a, when the MI was associated with PCI, with an increase of creatinine kinase-MB or cardiac troponin T more then three times the 99th percentile of the upper reference limit, and MI Type 4b when the MI was associated with stent thrombosis as documented by angiography or at autopsy. TLR was defined as repeat revascularisation within the stent or within the 5-mm borders proximal and distal to the stent on follow-up angiogram. TVR was defined as repeat revascularisation within the treated vein graft. Stent thrombosis was defined as angiographic documentation of stent occlusion, unexplained death within 30 days, or target vessel myocardial infarction at follow-up8.
Bleeding was assessed according to the classification of the thrombolysis in myocardial infarction (TIMI) study6. High surgical risk was defined as a EuroSCORE (European System for Cardiac Operative Risk Evaluation) >6 9.
Endpoints
Primary endpoint was to achieve antegrade TIMI flow 3 at Stage 3. Secondary clinical endpoints included the incidence of MACE and bleeding in hospital and at follow-up (30 days, six months and two years).
Statistical analysis
Continuous variables were expressed as mean±SD and categorical variables were presented as counts and percentage. A two-tailed p value <0.05 was considered significant for hypothesis testing. All analyses were performed using SPSS version 12 statistical software (SPSS Inc., Chicago, IL, USA).
Results
Mean age was 69±8 years and 18 out of 20 patients were male. Diagnosis at admission was unstable angina in 13 patients (50% were Braunwald class IB) and NSTEMI in seven. The studied population included patients with high comorbidities such as diabetes mellitus (45%), low left ventricular ejection fraction < 50% (60%), renal failure (35%) and a high surgical risk with a Logistic EuroSCORE of 26±18% (range: 4-65%). The mean graft age since bypass operation was 12±5 years. Previous mammary artery bypass was used in 70% of cases. The majority of SVG treated were on the obtuse marginal artery (45%). All but one SVG were occluded proximally, and the duration of occlusion was 59±80 days (1-285).
Procedural and in-hospital outcome
Procedural characteristics and outcomes are shown in Tables 1 and 2, while Figure 2 shows the angiographic result in each step.
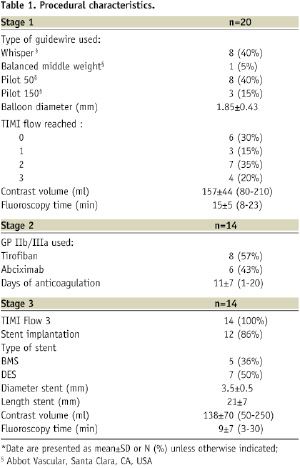
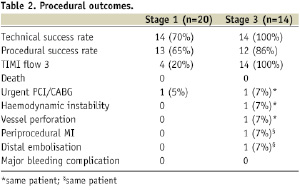
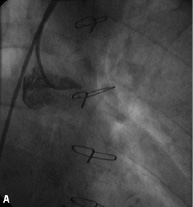
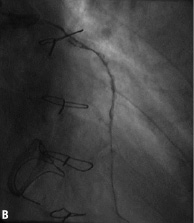
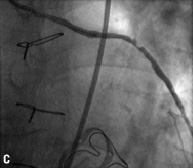
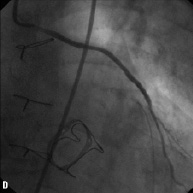
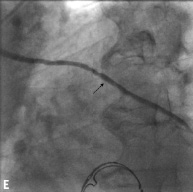
Figure 2. Angiographic results in each step during multistage procedure: A) total occluded SVG; B) recanalisation of graft with undersized balloon; C) focal lesion after anticoagulant period; D) single stent implantation; E) one year follow-up.
Technical success rate was 70%. Stage 1 was unsuccessful in six cases due to the inability to cross the occlusion with guidewire (five patients) and to failure of balloon dilatation (one patient). The total occlusion (Figure 2A) was “blindly” crossed with 0.014” hydrophilic and floppy tip angioplasty guidewires and the entire length of the vein graft was then predilated with undersized balloon with mean diameter of 1.85±0.43 mm. Predilatation did not establish definitive antegrade flow in any of the vein grafts (Figure 2B), but allowed for infusion of sufficient contrast to identify a partial recanalisation with large thrombus burden with intragraft clots greater then 30 mm. The recanalisation of the vessel was followed by the anticoagulant therapy (the second stage). Tirofiban upstream was administered in ten patients (50%) and Abciximab in six patients (30%). The mean anticoagulation time was 11±7 days (range 1-20 days). Stage 3 revealed the patency of all 14 saphenous vein grafts (100%) with TIMI flow 3. In three patients, an additional period (5-7 days) of anticoagulation was needed to obtain a further significative reduction of the thrombus. A focal lesion was present in 12 patients (86%) and was treated with stent implantation (Figure 2C and D). Seven drug eluting stents (50%) and five bare metal stents (36%) with a mean diameter 3.5±0.5 mm and mean length 21±7 mm were implanted. In the other two cases (14%) the SVG had no residual lesion and the procedure was completed. Total procedural success rate was 55% and the in-hospital MACE rate was 10%. We observed one periprocedural MI (5%) due to distal embolisation leading to a significant myocardial damage (peak CK-MB:22 ng/ml –x4 compared to baseline levels). Two urgent revascularisations occurred. One patient had had an abrupt rupture of SVG during stent deployment complicated with acute cardiac tamponade which required an urgent pericardiocentesis and urgent CABG surgery, and the other one had a subacute occlusion of the vein graft (at stage 2, on the third day from the index procedure) treated by urgent percutaneous revascularisation. A significant increase of Troponine T (peak 5.39 –x5 compared the baseline levels), but not of CK-MB, occurred in this patient. No death, in-hospital late closure or major bleeding complication requiring transfusion occurred during the staged procedure treatment.
Clinical follow-up
Short and long-term outcomes are presented in Table 3.
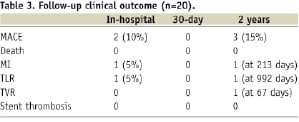
No MACE was observed at 30-day follow up. The overall incidence of MACE was 15% at two years. Angiographic follow-up was available for seven patients (35%). TVR was performed in one patient who had recurrent angina symptoms 67 days after the index procedure and TLR was performed in one patient who developed in-stent restenosis 992 days after the index procedure. One patient had a NSTEMI at 213 days of follow up due to a progression of disease on a different vessel.
Discussion
The present study describes the immediate and long term clinical outcomes of 20 patients with non-ST segment elevation acute coronary syndromes who underwent revascularisation of totally occluded SVG using a multi-staged procedure. The results of this preliminary study suggest that this novel approach can be successfully used to decrease intragraft thrombus prior to definitive stent placement, reducing the incidence of distal embolisation and improving the long term outcome in a particularly high risk profile population. Prior myocardial infarction, three vessel and/or diffuse coronary disease, advanced age, low ejection fraction and impaired renal function were in fact common characteristics of our population. Usually the revascularisation of the native vessel is a better option than opening an occluded, degenerated graft but there are instances when, due to presence of diffuse disease, tortuosity or high calcium, the treatment of native vessel is not feasible.
Percutaneous treatment of SVG, especially occluded graft, is difficult, compared to the angioplasty of native coronary arteries, due to the peculiar morphology and structural characteristics of SVG atherothrombotic plaques previous described. Currently, the common percutaneous treatment of SVGs is stent implantation with the use of embolisation protection devices. Several studies have confirmed that use of BMS in SVGs improves outcomes compared with balloon angioplasty10, and recently the safety of DES in SVG was also reported11,12. The 2005 European guidelines recommended contemporary use of embolic protection device during PCI of SVG to avoid no reflow phenomenon (Class of Recommendation I, Level of Evidence A)13.
In the past years different solutions were used for the treatment of totally occluded SVGs arising different results in term of procedural success, ranged from 65%14 to 70%15,16, with high periprocedural complication such as death (6,5%)15, distal embolisation (11-17%)15-17 and significative increase of total CK creatinine kinase (up to 43%)16.
Our results are similar to what is reported so far in the literature, with a technical success rate at Stage 1 of 70%, but the second stage (prolonged anticoagulant therapy) allows to decrease thrombus burden reducing distal embolisation phenomenon during stent deployment. Moreover, the degeneration in the entire length of the vein graft could yield to multiple stents implantation with an increased risk of stent thrombosis. Our results suggest that the reduction of thrombus burden can also avoid the multiple stent implantation in a degenerated graft, detecting a focal lesion treated with single stenting.
Two-year follow up outcomes are good especially considering the high risk profile of our population with overall incidence of MACE of 15%.
No death or fatal myocardial infarction occurred. This could be explained by thrombus removal and short stent implantation. Recently, Pucelikova et al14 demonstrated that the presence of thrombus before the procedure and total stent length per graft were independent predictors of MACE at 1-year follow-up.
The major difficulty of this procedure is to decide the period for anticoagulation therapy because it is directly related to the amount of thrombus burden. Usually, we made an angiographic control the day after the recanalisation in order to evaluate the response to the anticoagulant therapy and then decide the anticoagulation time. More the intragraft clots, more the anticoagulant time is needed. In our experience the mean anticoagulation time to achieve a significant thrombus reduction was 11±7 days (range: 1 to 20 days). This could be considered a limit of our study, because we are unable to give guidelines with respect to the anticoagulation time. Moreover, the small size of the sample, the selection of the patients and the single centre experience represent the other important limitations of the present study. Our date showed good results when the occlusion of SVG was recent (an occlusion <3 months evaluated by clinical and angiographic date), but we cannot extend these considerations for the treatment of all kind of SVG occlusions, in particularly for chronic SVG occlusions.
Conclusion
This real-world single centre experience is the first report to describe the use of a stage procedure for the treatment of SVG occluded. The results of this preliminary study suggest that a three stage procedure can be used successfully in order to decrease intragraft thrombus prior to definitive stent placement, reducing the incidence of distal embolisation and improving the short and long-term outcome in a particularly high risk profile population.

