Abstract
About 5% of patients annually have no acceptable therapeutic option because of diffuse coronary disease without a discrete target for angioplasty, stenting or surgical bypass. Retrograde perfusion of the myocardium via the venous system was successfully performed by cardiac surgeons before the widespread use of coronary artery bypass grafting and has been applied in “ungraftable” patients since. Percutaneous in situ venous arterialisation (PICVA) follows this surgical experience, using a series of unique catheter-based devices to arterialise isolated segments of coronary vein to provide retrograde perfusion of the ischaemic myocardium. In a series of animals this procedure had been shown to reduce ischaemia and myocardial damage in an infarction model. In the PICVA European Safety trial, the first eleven percutaneous applications of catheter-based, coronary bypass in humans revealed feasibility of the technique in highly symptomatic, non-option patients. Despite two deaths and six failures to create a connection, we continue to believe that upon further development, the catheter-based veno-arterial coronary bypass can become an option in inoperable and undilatable patients.
Introduction
Ten years after bypass surgery about 40% of the vein grafts and 20% of the arterial grafts are occluded1. Percutaneous intervention in chronically occluded grafts has traditionally been severely limited by guidewire access to the distal native vessel, embolisation of the subtended myocardium during revascularisation and a high rate of restenosis and re-occlusion2. Most of the native vessels are chronically occluded as well, and may be the preferred target for a percutaneous intervention. However, the success rate in these very old occlusions of often diffusely diseased and calcified vessels may be considerably less than 50%3. A second bypass operation might be considered, however the risk is increased and often unacceptable.
These so-called “no-option” patients frequently have diffuse coronary disease without a discrete target for angioplasty, stenting or surgical bypass. It has been estimated that more than 5% of all patients each year may fall beneath the “no-option” rubric4.
Gene therapy and laser revascularisation strategies to create new blood vessels in ischaemic myocardium have not yet been convincingly successful. Myocardial tissue requires significant arterial inflow, not obviously provided by these techniques. Therefore, alternative routes and techniques to improve myocardial perfusion in these patients appears warranted.
In 1927, Wearns was able to show that upon blockage of the coronary veins, 90% of the venous blood will be drained by the Thebesian system; thus an arterialisation of the veins should be possible, without risking detrimental congestion.
As shown by several surgical series, arterialisation of the coronary veins indeed has the potential of providing strong arterial in-flow to a severely ischaemic region and to improve the symptoms of patients with severe angina5-7. For example, in the Park series, six patients with diffuse LAD disease received a Lima-graft to the anterior interventricular vein (AIV) and all remained free of angina after one year8.
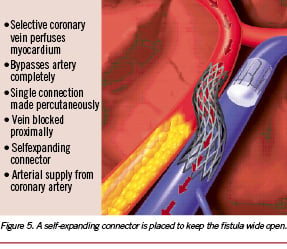
Recalling this possibility for venous retro-perfusion, a catheter technique, percutaneous in situ venous arterialisation (PICVA) and percutaneous in situ coronary artery bypass (PICAB) were developed for arterialising the in situ coronary veins from an adjacent proximal coronary artery, blocking the proximal vein, and thus forcing retrograde venous perfusion (Figures 3, 4, 5). The feasibility of this technique was explored in a large number of animal studies, and it was shown in a controlled pig trial that PICVA is able to reduce infarct size and mortality9. (Figure 1)
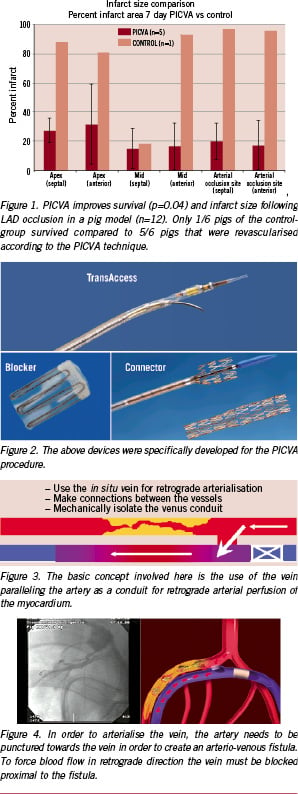
The fundamental PICVA procedure
In humans, the procedure was first described by S. Oesterle, N. Reifart et al in 200110. To perform PICVA, a series of specialised catheters and implantable devices (TransVascular, Inc, Menlo Park, CA, USA) are required. These catheter-based devices can be divided into four categories (Figure 2):
1. Coronary sinus and sub-selective guiding catheters: developed to access the coronary sinus and then maintain a stabilisation platform for devices to be introduced into the coronary sinus and the branching coronary venous system.
2. TransAccess™ catheters: unique devices designed to introduce a guidewire from one vessel to another (from artery to the adjacent vein and vice versa) with precision and control. The TransAccess™ catheters are guided through vascular segments using fluoroscopy, vessel-to-vessel targeting is performed using an integrated intravascular ultrasound guidance system.
3. Flow directing / blocking devices and delivery systems: “Blocker”; for deliberate total occlusion of targeted venous segments – preventing shunting of arterial inflow back to the coronary sinus and into the right atrium.
4. Channel creation and maintenance devices: “Connector”; designed to be delivered over a 0.014» arterio-venous guidewire and used to create a permanent conduit between the vessels.
Current experience
A phase I safety trial of PICVA, was started in November 1999 by S. Oesterle, N. Reifart and K.E. Hauptmann at the Krankenhaus der Barmherzigen Brüder in Trier, Germany. The protocol for this Phase I study had been reviewed and approved by the Freiburger Ethik Kommision International. All patients gave informed consent for the PICVA procedure. The study was limited by protocol to those patients with severe angina and no reasonable option for either angioplasty or surgical revascularisation.
The present data stems from a first generation approach and prototype devices. Eleven patients had been treated (10 at the Krankenhaus der Barmherzigen Brüder in Trier, Germany and one at the Universitätsklinik Essen, Germany) and were reported in 200311.
Interventional procedure
The patients receive minimal sedation and are given a bolus of heparin to achieve an activated clotting time (ACT) of between 280- 300 seconds. A 9 Fr sheath and a 16 Fr introducer sheath are placed in the right femoral artery and vein, respectively. An initial angiogram and limited coronary IVUS exam (EndoSonics Corp, Rancho Cordova, CA, USA) are performed to reveal a suitable parallel vein within 3 mm of the proximal portion of the coronary artery. Using a standard 5 Fr AL-1 angiographic catheter, the coronary sinus is cannulated and a 0.035” hydrophilic guidewire was introduced into the vein. Once the wire is positioned in the anterior interventricular vein (AIV), a 16 Fr CS-2 coronary sinus guide catheter and introducer (TransVascular, Inc, Menlo Park, CA, USA) is advanced over the .035” guidewire and positioned in the coronary sinus. Through the coronary sinus guide, a sub-selective delivery catheter and introducer (TransVascular, Inc, Menlo Park, CA, USA) are delivered deeper into the venous system, providing direct access to the region between the great cardiac vein and the AIV. Through a 9 Fr VL-3.5 Voda guide catheter (Boston Scientific-Scimed, Maple Grove, MN, USA), positioned at the ostium of the left main coronary artery, a 6 Fr TransAccess Catheter (TransVascular, Inc, Menlo Park, CA, USA) is advanced into the proximal coronary vessel. The vein is then identified utilising the ultrasound imaging array on the TransAccess catheter and a targeting pointer is positioned toward the adjacent vein. The nitinol needle is advanced through the arterial wall into the vein and a 0.014” high torque floppy exchange guidewire (Guidant/Boston Scientific, Santa Clara, CA, USA) is passed through the needle and positioned in the distal AIV. The needle is retracted and the TransAccess catheter is removed, leaving the exchange wire between the proximal coronary vessel and the AIV. A 1.5 mm and 3.0 or 3.5 mm PTCA balloons are used to pre-dilate the channel between the vessels. The channel is created in the sub-adventitia and haemorrhage into the pericardium should not occur. Following channel dilatation, a self-expanding TransVascular Connector (arterial diameter: 3.5 mm, venous diameter: 4.0 mm) is introduced into the channel and positioned between the vessels. The connector is deployed by a retractable sheath delivery system. The channel is post-dilated with a high-pressure angioplasty balloon and then inspected for proper dimension and apposition with IVUS. The channel is created in the sub-adventitia, and haemorrhage into the pericardium should not occur. To prevent shunting of blood to the coronary sinus, a TransVascular Venous Blocker is delivered through the sub-selective venous catheter and released at approximately the AIV-GCV junction.
Results
PICVA was attempted in 11 patients. In six patients, the adjacent vein could not be adequately targeted for successful needle and wire delivery. Variations in anatomy, including vessel crossovers and unexpected distances (greater than 6-8 mm), introduced an element of parallax and ambiguous trajectories for nitinol needle delivery. Furthermore, significant plaque burden in the proximal LAD obscured far-field imaging and occasionally impeded the delivery of the TransAccess catheter. In five of these cases the procedure was aborted without significant complications. In one patient, however, a perforation in the venous system near the coronary sinus resulted in tamponade requiring emergency surgical repair. This patient ultimately received a surgically placed aorto-saphenous graft to the anterior interventricular vein, with ligation to permit distal retro-perfusion.
PICVA was successfully completed in five patients. Two of the five patients had catastrophic complications and died within 48 hours of the procedure. While one of these cases may have been further complicated by acute thrombosis within a stent placed in the LAD proximal to the PICVA channel, both patients displayed extensive epicardial haemorrhage. Problems with blocker positioning were encountered in both cases. Undersized blockers were inadvertently selected, and both subsequently migrated towards the coronary sinus following delivery. Redeployment of these blockers, followed by additional blocker deployments, may have contributed to the trauma. Our post-mortem analyses suggest that haemorrhage from the vein and intramyocardial haemorrhaging from a greater than intended retro-perfusion area, played a significant role in both these fatalities. Of the remaining three cases, all patients experienced an improvement in their anginal symptoms. One of these, a 53-year old, Type II insulin-dependant diabetic man, remains free from chest pain (CCS angina class 0) at a follow-up of 2.5 years, despite the fact that he presented at baseline with class 3 angina. Quantitative angiography at three months for this patient revealed a patent PICVA graft with a minimal luminal diameter measuring 2.04 mm within the anastomotic channel. The second patient’s angina was reduced from class 3 pre-treatment to class 0 post-treatment; however, three month angiographic follow-up revealed a closed PICVA channel and a modest rise in angina to CCS angina class 1. Her anginal class was reported as class 1 at two years post-procedure. During the case involving the third patient, there was a perforation of the anterior interventricular vein (AIV), necessitating pericardiocentesis and deployment of a stent/graft (Jomed Inc, San Diego, CA, USA) from the LAD to the AIV. Post-procedurally, a Q-wave myocardial infarction was detected, presumably due to the exclusion of a side-branch distal to the PICVA channel. This patient recovered uneventfully and was discharged from the hospital. At one month follow up the stent/graft was fully occluded, and the patient’s anginal status was reported as class 1. A more detailed description of these results has already been published earlier11.
Discussion
The current technique for percutaneous coronary bypass was hampered by failures and complications and is not likely to be introduced into clinical practice, however, the the PICVA experience – both in animal and men – is encouraging and teaching us with respect to several aspects:
1) The procedure revealed that the surgical experience to establish improved myocardial perfusion through arterio-venous coronary connections can be duplicated using a catheter-bases percutaneous approach.
2) Despite inconstant venous anatomy, an arterial to venous connection should be feasible at the mid-LAD-level.
3) Working in the very proximal LAD is confounded by inadequate proximity of the AIV.
4) Multiple needle passes from the artery can be tolerated without clinical consequence.
5) Deployment of blocking devices in coronary veins requires accurate sizing and precise placement.
Upon further development of visualisation and refined devices, percutaneous bypass approaches should become feasible, safe and efficacious procedures in properly selected patients. On the other hand, the success of opening chronically occluded coronary arteries has increased in the past years due to refined specific material and advanced techniques like the parrallel wire technique12 and retrograde approach13,14. However, patients with a lack of conventional options might well be candidates for our innovative and unorthodox approach which will hopefully see a revival in the near future.
Acknowledgments
The author acknowledges the seminal contribution of Josh Makower, MD and Stephen Oesterle MD, who conceived the principles for PICVA and PICAB as well as Eugen Hauptmann, MD, and Ted Lamson, who deserve major credit for the work at the Krankenhaus der Barmherzigen Brüder, Trier.

