Abstract
Coronary revascularisation should be considered as a healthcare process rather than a series of episodic interventions. At a time when the number of surgical and interventional procedures worldwide continues to increase, secondary coronary revascularisation appears as an unavoidable subject. Atherosclerosis progression, long-term failure of surgical grafts or stents, and patient profile contribute to the increased risk of secondary revascularisation. The absence of a grouping category, however, has contributed to suboptimal implementation of evidence-based knowledge on the subject, which is scattered in the literature and scantily covered in clinical practice guidelines. Assembling a critical mass of expertise in the field results mandatory for comprehensive patient management and for highlighting avenues for future research. Knowledge sharing between physicians, interventionalists and surgeons appears indispensable to reduce unilateral decision-making. Awareness of all health professionals about the likelihood of repeated revascularisation appears as the first step towards a process-oriented and holistic management of patients requiring coronary revascularisation.
Introduction
A revisited history of coronary revascularisation should necessarily include the words “short” and “successful”. Coronary artery bypass grafting (CABG) was first performed in 1967; forty years later, more than 800,000 CABG procedures per year are performed worldwide. Percutaneous coronary interventions (PCI) was developed only 10 years after the introduction of CABG, at a time when revascularisation was the province of the cardiac surgeon; by 1990 the number of PCI procedures, performed by cardiologists, exceeded that of CABG interventions per year, and by 2007 it was estimated that more than 2.5 million angioplasty procedures were performed worldwide, using more than 3.5 million stents.
During its fast development, numerous modifications were introduced in both types of revascularisation techniques. In the surgical field, arterial grafts were introduced to avoid the aggressive venous graft atherosclerosis, which seriously compromised long-term conduit patency. Off-pump procedures decreased complications associated to aortic clamping and cardiopulmonary bypass. Mini-thoracotomy approaches were developed, and robotic surgery was also introduced. In percutaneous revascularisation, the advent of coronary stents contributed to achieve larger and smoother endoluminal surfaces and to decrease acute vessel occlusion. Stents also contributed to decrease long-term failure due to restenosis, particularly with the introduction of drug eluting prostheses. Atherectomy techniques enabled the possibility of working in unfavourable substrates, such as heavily calcified vessels. Thrombectomy and anti-embolic devices were developed to work in situations with high risk of coronary embolisation during PCI, and jointly with adjunctive antithrombotic treatment, improved PCI in thrombus-containing vessels. Primary PCI became the treatment of choice in acute myocardial infarction. Endoluminal guidance of PCI became possible with intracoronary ultrasound and physiology techniques.
With the rapid development of PCI, its use was soon expanded from single, focal stenosis, to more complex subsets of lesions and patients. Once the frontier of treating multivessel disease with PCI was crossed, many voices claimed for the need of randomised clinical trials (RCT) comparing the safety, efficacy and efficiency of PCI with that of CABG. By 2003, 13 RCTs comparing PCI versus CABG in multivessel coronary revascularisation had already been concluded1, but their conclusions were soon challenged. This was due to relentless developments in interventional techniques: balloon angioplasty had been superseded by bare metal stents first, as these stents themselves were by drug eluting stents sometime later.
But the history of coronary revascularisation presents also quite relevant aspects belonging to the professional sphere that must be briefly introduced for a more complete understanding of its evolution. Thus, it should be mentioned that, as a result of conflicts of interest involving spheres of competence, a strong competitiveness between surgical and percutaneous revascularisation teams dominated long segments of the described development period, and this led to a litigious climate between interventionalists and surgeons that made cooperative experiences, such as hybrid revascularisation, the exception and not the rule2. At the time of crossing into the new century, the achieved evidence based on RCT´s and meta-analysis enabled the writing of recommendations in clinical practice guidelines (CPG) as to which technique was most indicated in various clinical scenarios of primary coronary revascularisation. However, the most recent trials make foreseeable that a vivid debate on this topic will continue, at least in the near future3. Although one of the main items of current discussion is the difference in repeat revascularisation rates between both techniques, a comprehensive approach to repeat revascularisation has not been performed yet. This will be discussed over the following paragraphs.
Repeated coronary interventions: an issue at the beginning of the 21st century
There is limited available data on current secondary coronary revascularisation rates. Data from the European Heart Survey on coronary revascularisation collected on 7,769 patients in 2001-2 revealed that of the 4,442 patients undergoing surgical or percutaneous revascularisation, 14% had a previous history of CABG and 34% of PCI4. More recent information, obtained in the New York State registry already in the DES era (2003-2005) revealed an overall reintervention rate of 36% within the first 18 months after coronary interventions (Figure 1), with most cases being repeat PCI procedures after a drug eluting stent implantation (28%)5. Figure 2 shows data from 22,426 PCI procedures performed at our institution between 1985 and 2009, with figures of all PCIs and of those performed in patients with prior history of PCI or CABG. The tremendous increase in procedures that took place over the last 24 years reflects both the increased demand and the inclusion of new expanded indications of PCI (multivessel disease, acute myocardial infarction, etc.). The approximate date when relevant developments were introduced is displayed below the graphic. The percentage of patients with prior interventions (surgical or percutaneous) undergoing PCI remains pretty stable since 1990, being 34% within the last four years.
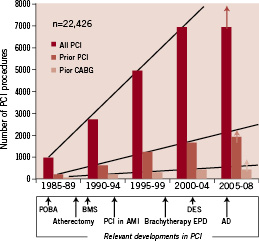
Figure 1. Institutional trends in percutaneous revascularisation, as contemplated from 22,426 percutaneous revascularisation (PCI) procedures performed at our institution (Hospital Clinico San Carlos, Madrid, Spain) between 1985 and 2009. Data corresponding to all procedures and to those performed in patients with prior history of PCI or CABG are shown separately. The approximate date when relevant developments in PCI were introduced is displayed below the graphic. The percentage of patients with prior interventions (surgical or percutaneous) undergoing PCI remains pretty stable since 1990, being 34% within the last four years. The last lustrum (period of five years) is incomplete, and only a 4-year period is shown (therefore the arrows predicting figures by the 5th year).
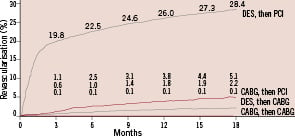
Figure 2. Rates of secondary coronary revascularisation within 18 months after surgical (CABG, n=7,437) or percutaneous (PCI, n=9,963) primary coronary interventions in the New York State registry between 2003 and 2004. Data corresponding to four different modalities of secondary revascularisation is shown in different curves. By far, the most frequent modality of secondary revascularisation during this period was PCI after initial percutaneous revascularisation. This was followed by PCI after initial CABG. Hannan et al N Engl J Med 2005;352:2174-83. Copyright © 2008 Massachusetts Medical Society. All rights reserved.
The possibility of undergoing more than one coronary intervention has increased substantially due to several factors. First, the ageing of the population increases not only the number of cardiovascular patients, but also the possibility of disease progression or surgical graft failure in those with previous coronary revascularisation. Thus, age at the time of the first revascularisation procedure influences the chance of undergoing secondary revascularisation in the future, with younger patients being more likely than older ones to undergo repeat procedures6. Second, a continuous increase in the accessibility to coronary revascularisation techniques has taken place over the last 20 years, largely due to the creation of new cathlabs but also due to the development of new surgical units or to an increase in the activity of pre-existing ones. Third, incomplete revascularisation, either as a result of culprit lesion PCI or CABG, over the long term causes more repeat coronary interventions6. Finally, in spite of the introduction of DES, the absolute figure of repeated revascularisations for restenosis will increase as a result of the growing number of patients treated with PCI.
A considerable amount of evidence has been gathered on different aspects of diagnostic and therapeutic management of patients undergoing repeat coronary interventions. It is remarkable, however, that this accumulated knowledge is scattered in the literature. No recognisable attempts to bring together, in a comprehensive fashion, all the problems posed by these patients, nor the proposed solutions, have been made. Even bibliographic search strategies for this topic are non-existent, and systematic reviewing requires the use of terms such as “reoperative”, “redo”, “repeat”, “bypass”, “restenosis”, etc., just to name a few.
In a way, the lack of a recognisable category grouping all the available knowledge on this topic mirrors the above-mentioned divisions and lack of synergy between surgical and interventional teams discussed above. Decision-making in redo coronary procedures, either surgical or percutaneous, has been frequently taken in the context of a generalised confrontation of surgeons and interventional cardiologists. Besides, relevant clinical practice guidelines have not addressed secondary revascularisation as a topic in its own8-10. Evidence-based planning of a second revascularisation results are far more complex than in primary interventions.
In this void of evidence, decisions on secondary revascularisation have been frequently taken ad hoc and unilaterally. One of the main risks of this attitude is that it leads to episodic care, which lacks a longitudinal perspective and does not contemplate the potential of future coronary revascularisations. From this point-of-view, coronary revascularisation should be considered as a care process rather than a series of single interventions. This has obvious implications for decision-making and patient follow-up. Key aspects like the enforcement of secondary prevention measures should be considered part of the process of coronary revascularisation. A renewed multidisciplinary approach, untied to former conflicts, must be promoted11. The current scenario of changes in cardiovascular medicine and health care may provide an opportunity undertaking process re-engineering of coronary revascularisation12.
Cardiovascular risk factors, atherosclerosis and secondary coronary revascularisation
What is wrong in patients requiring multiple interventions? Why do they have a higher risk after a first coronary intervention6,8,13? In answering these questions we should remember that the generalisation of PCI, the fastest and more frequent method of revascularisation, has reinforced a pragmatic approach to the management of coronary artery disease; as a consequence of this, not infrequently, the complexity of coronary atherosclerosis is overlooked, leading to erroneous judgements on the impact of revascularisation on patient’s prognosis. One potential (and rather common) fallacy of such an approach would be the proposal that the revascularisation of two vessels in patients with triple vessel disease means improving cardiovascular risk to the level of that of patients with single vessel disease. Even if the number of patent vessels becomes the same after intervention, it is easily understood that the former patients probably have a more aggressive underlying atherosclerotic process, which, in the long term, will imply a worse prognosis than those with single vessel disease.
Patients requiring multiple coronary interventions provide a similar scenario. They present, in statistical terms, more frequent and concomitant cardiovascular risk factors that trigger aggressive atherosclerosis6,14. At a systemic level, this translates into a higher prevalence of extracardiac complications such as renal insufficiency and stroke; and at a cardiac level into more frequent episodes of infarction and lower LV ejection fraction. Besides this, they present a larger atherosclerotic burden in their coronary vessels which results, among other causes, from disease progression during the time elapsed from the first intervention, leaving fewer options for surgical and percutaneous reinterventions6,13.
To expand the complexity of this issue, long-term failure of the primary revascularisation may result from achieving suboptimal procedural results in the context of extensive, diffuse atherosclerosis. Small vessel calibre and vessel calcification, for example, are at the same time determined by the presence of cardiovascular risk factors, and also important determinants of optimal stent expansion and performance of adequate anastomoses of surgical grafts.
Therapeutic aspects of secondary revascularisation
The issue of deciding the technique of choice, surgical or percutaneous, in patients with prior coronary interventions is obviously a key one. Relevant clinical practice guidelines have barely covered the issue of secondary revascularisation. A recent (2009) document endorsed by several American scientific societies17 introduces a wider discussion of repeated revascularisation in patients with previous CABG; this constitutes a relevant step, although a comprehensive revision of the topic is still required.
Many items deserve consideration given this void of evidence. It appears of great importance to initiate a comprehensive approach to decision* making in secondary revascularisation; patients with prior CABG have been systematically excluded in CRT comparing primary CABG and PCI in multivessel disease, and therefore no information on this subgroup is available. Likewise, information on current patterns of secondary revascularisation should be obtained and analysed. Reviewing the available evidence on the interference of revascularisation techniques in the long-term appears as an important task: this might be the first step towards creating awareness among the operators on the importance of contemplating primary revascularisation procedures in the scope of a cardiovascular biography, and not of an isolated cardiovascular event. The following paragraphs will discuss briefly these and other aspects of surgical and interventional reintervention on patients with previous CABG and PCI.
SECONDARY REVASCULARISATION IN PATIENTS WITH PRIOR CABG
Previous CABG is a frequent context for secondary revascularisation, increasing dramatically in the second decade after CABG6,8,13,18. The number of redo CABG procedures exploded around 19853,18, which was followed by a more conservative and critical attitude that, later, was superseded by PCI procedures13. Retrospective studies have demonstrated that both approaches have a significant higher risk than in the context of primary revascularisation. Compared with primary multivessel revascularisation, 5-year mortality has been found to be virtually twice as high in patients undergoing secondary revascularisation, either percutaneous (25 vs. 16%) or surgical (21 vs. 14%)19 (Figure 3). This is in agreement with surgical series that consistently identified redo CABG as a predictor of risk in coronary artery surgery6,18. Cumulative advances in perioperative management, such as minimally invasive incisions, new modalities of myocardial protection and off-pump intervention, may have contributed to a decrease in the risk of redo CABG, to the point that some authors propose that reoperation itself is not longer a predictor of poor outcome after CABG20.
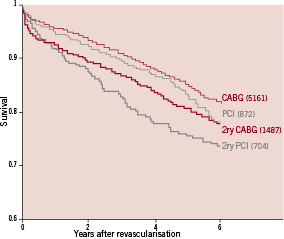
Figure 3. Unadjusted survival curves from PCI and CABG cohorts after primary multivessel revascularisation (CABG and PCI, thin lines) and secondary revascularisation (re-CABG and re-PCI, broad lines) from a large volume centre (Cleveland Clinic, Ohio, USA), showing the poorer outcome of patients undergoing secondary revascularisation, irrespective of the technique used. The graphic has been built by merging data from two separate reports from the same institution (references 13 and 19). The number of patients included in each cohort is shown between brackets.
Decision-making on the modality of secondary revascularisation in patients with prior CABG appears to be influenced by a number of anatomical and clinical features15. Surgical reintervention has been found to be preferred over PCI in patients at higher risk, with fewer functional grafts, more chronic total occlusions, and lower systolic function. Conversely PCI was the technique of choice in patients with patent LIMA and a suitable coronary anatomy. The benefit of choosing any of these modalities, however, appeared to be limited, since prognosis was mostly affected by age and LV ejection fraction.
Evidence on how referral physicians and patients influence the modality of secondary revascularisation after CABG can be found in the registry of the AWESOME (Angina With Extremely Serious Operative Mortality Evaluation) trial21, the only dedicated CRT to secondary revascularisation after CABG to date. This study demonstrated that physicians and patients opt for PCI by a 2:1 margin over redo-CABG. For the design of trials comparing surgical and percutaneous modalities of secondary revascularisation, the AWESOME trial constitutes a warning on the difficulty in convincing physicians or patients to permit random allocation of high-risk cohorts of patients: for the frustration of the AWESOME investigators, and despite all the efforts involved in this large USA trial, most medically refractory patients with prior CABG were allocated either by physician direction or patient choice.
A similar trend to refer CABG patients to secondary treatment with PCI can be also observed in more contemporary data obtained in New York State5. Crossover between surgical and percutaneous modalities in re-intervened patients occurred in 7% of patients: the chances of undergoing PCI as a secondary procedure after surgery was more than twofold that having CABG as a reintervention after PCI. In this regard, it should also be remembered that surgeons might feel reluctant to expose patients to the risk of damaging the existing grafts, a concern that provides a partial explanation to why patients with multiple grafts undergo less surgical secondary revascularisation in spite of presenting ischaemia.
Another example of the complex interplay of both types of revascularisation techniques can be found in percutaneous treatment of patients with previous CABG. Saphenous vein graft attrition is caused by an accelerated form of atherosclerosis which behaves in quite a different way than native coronary atheroma: the outcome of PCI is not improved by concomitant use of IIb IIIa inhibitors, there is a high risk of non-reflow phenomenon, the probability of rupture is higher than in native vessels, restenosis rate after stent implantation is high. In this microcosm of all possible PCI misadventures, as it has been described, major advances have been performed, bringing light, but also, unfortunately, projecting shadows. Thus, while the introduction of intracoronary embolic protection devices (EPD) constituted a major breakthrough in SVG PCI, with multiple RCTs demonstrating the benefit of their use (class IA recommendation in clinical practice guidelines9), application of EPD in real life is far from being universal23. Failure to implement EPD usage in SVG PCI cannot be justified by technical problems, such as the presence of bifurcations, small vessel diameter or aorto-ostial location. Likewise, while the introduction of drug eluting stents anticipated a solution for the high restenosis rate observed in SVGs, the long-term results of the only RCT comparing DES and BMS in these conduits have caused major concerns as to its safety24. Even diagnostic issues are complex: interpretation of FFR in SVG is not easy for the average cardiologist, as it is deferring treatment of an SVG stenosis on the grounds of FFR in the context of accelerated graft atherosclerosis25.
SECONDARY REVASCULARISATION IN PATIENTS WITH PRIOR PCI
The outcome of CABG in patients previously treated with PCI was scarcely addressed until recently. In a way, a liberal use of stents was widely accepted as a way to defer surgical revascularisation, and the potential occurrence of longitudinal interactions between coronary interventions has probably been underestimated. Several studies based on a large population of CABG procedures have reported on the predictive value of prior PCI in the development of major cardiac events, including death, after coronary surgery26. These findings deserve a meticulous analysis since, upon confirmation, could potentially have either a biological or an attitudinal origin. Among the former would be the (speculative) detrimental effect of stents on distal coronary bed. Among the latter, a liberal use of stenting, leading to situations as the so-called “full metal jacket”, which might result in fewer surgical grafts implanted at the time of the operation. It appears reasonable to think that operator awareness of these interactions and of the probability of future need of CABG, so rarely expressed today in an explicit fashion in any document, may lead to a shift in attitude at the time of planning coronary stenting, avoiding just this type of episodic care. Likewise, such attention might foster the application of new technologies, such as drug eluting balloons27 or resorbable stents28, which may not compromise surgical access to the coronaries in case of disease progression.
By far, nowadays, repeated percutaneous revascularisation is the most frequent modality of secondary revascularisation. This is a consequence, first of the high number of PCI procedures performed, but also of self-referral within the domain of the cardiologist29. Data from RCT on the treatment of multivessel disease clearly illustrate this pattern. To provide the latest example, in the SYNTAX trial, secondary revascularisation was performed with PCI within the first year after randomisation at a similar rate (80%) in cases allocated to DES and CABG arms, which presented secondary revascularisation rates of 14% and 6%, respectively3. In general terms, the use of PCI in more complex subsets of patients and lesions, including those grouped under the term “off-label” indications, is likely to increase the need of repeat revascularisation.
Coronary stents constitute an enormous source of revenue for biomedical companies, with a predicted market in excess of $12 billion in annual sales by 2012 (Global Industry Analyst data 2008). Research in the field, therefore, is strongly driven by the industry, with the obvious risk of bias. Strategic decisions can be made aside from clinical evidence. Coronary brachytherapy, which proved to be effective for the treatment of stent restenosis in multiple RCT (degree of evidence IA9) was “buried alive” largely by manufacturers, who withdrew their systems soon after the advent of DES and before clinical evidence on their use for that particular indication was reached30. In spite of the disappearance of the technique from the clinical arena, many patients that underwent intracoronary brachytherapy, mostly for restenosis but also for other indications, will still require new coronary interventions during their lifetime. Since the effects of radiation on vascular biology might persist, information on the safety of new interventions using bioactive technologies (i.e. DES) in segments treated previously with brachytherapy, as well as operator awareness on this current lack of information, must be promoted.
There are different threats posed by stenting to repeat percutaneous procedures. Coverage of major side branches may cause an access problem if future interventions are required in that vessel. This might also occur after certain bifurcations techniques, such as stent crushing, particularly if final optimisation with balloon kissing was not (or could not be) performed. Ostial location of stents in the left main or right coronary arteries with partial deployment of the prostheses in the aorta may difficult catheter cannulation. In secondary revascularisation, information on underlying factors favouring restenosis after the first procedure should be obtained and, when feasible, corrected. Intraluminal imaging techniques, such as IVUS or OCT, may provide valuable clues as to whether adequate expansion, stent fracture or stent collapse has occurred31,32, and therefore should be considered in secondary revascularisation with PCI. Guidance of secondary revascularisation with these techniques might potentially result in much higher benefit for long-term results than in first procedures, a topic that should be addressed by dedicated studies.
Contemporary diagnostic methods in secondary revascularisation
Paralleling the evolution of revascularisation treatments discussed above, multiple diagnostic technologies have been incorporated over the last 20 years. Again, some of these techniques have generated professional competitiveness and conflicts of fields of competence, as it has been the case for magnetic resonance (MR) and multislice computed tomography (MSCT) imaging with radiologists and cardiologists.
Cardiac imaging plays an important role in assessing the results of coronary interventions, and in planning secondary revascularisation. However, at a time in which new imaging modalities are largely available, this aspect of diagnosis deserves a detailed discussion. Coronary arteriography has been the gold standard in the assessment of native coronary circulation, surgical grafts and implanted stents, and continues to be the most widely used imaging technique for this purpose. The strength of this procedure is that it provides high quality images. However, after coronary intervention, the diagnostic power of coronary angiography also diminishes, since selective catheterisation and complete opacification of surgical grafts is not always achievable. Furthermore, the technique has more associated risks, derived from patient profile and technical difficulties. Catheter manoeuvring may damage supra-aortic vessels during selective catheterisation of thoracic arteries. Given the presence of more extensive atherosclerosis in these patients, increased catheter manipulation implies a higher risk of cholesterol embolism. Procedure-induced kidney failure may occur as a result of this, but also as a result of the larger amount of contrast given during angiography and aortography. These risks are exacerbated in the high number of diabetic patients requiring secondary revascularisation.
In this context, a contemporary approach using MSCT as a front-line imaging technique in patients with prior CABG should be explored. Although in many cases MSCT would be followed by invasive coronary angiography, such approach would make possible a more selective study, with restrictive imaging of native segments in which the sensitivity and specificity of MSCT is lower due to vessel calcification, blurring, etc. Multislice CT–assisted intervention is already a reality in fields like magnetic navigation, and it is foreseeable that in a near future co-registration of MSCT and angiography would be available in many cathlabs, contributing to perform PCI based on MSCT images (Figure 4). Magnetic guidance of PCI hardware based on this information may prove of particular use performing percutaneous interventions through the tortuous and complex vessel anatomy found after CABG, as described in this issue by Ramcharitar et al33. With regard to surgical interventions, anatomical information, such as the relationship of cardiac structures and previous grafts to the sternum obtained with MSCT, is of obvious value in planning operative strategies and in formulating preventive measures, such as tailored modalities of sternotomy, or the instauration of cardiopulmonary bypass before it is performed34.
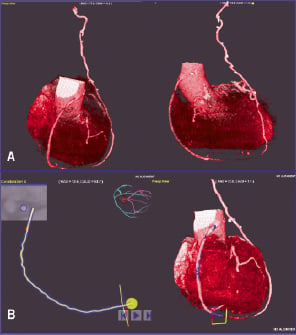
Figure 4. Multislice computer tomography (MSCT) constitutes a good example of how emerging diagnostic technologies provide new opportunities in planning and performing secondary revascularisation procedures. This figure shows a MSCT surgical graft reconstruction (LIMA to LAD, SVG to RCA) performed in the cathlab at our institution prior to a secondary percutaneous revascularisation with magnetic navigation. The information from a prior MSCT performed by the radiology department is used by the interventionalist to reconstruct the images (A), and to build the pathway that should be used by the magnetic navigation system during advancement of the magnet-tipped guidewire (B). It is foreseeable that MSCT workstations will be integrated in future cathlabs and operating theatres for this and many other functions. See text for a wider discussion on the subject.
In cases with previous stent implantation, diagnosis might also benefit from using other techniques than conventional angiography. This is the case in assessing restenosis, which currently is feasible with MSCT in large vessels, such as stented left main coronary arteries and surgical grafts (with the advantages, for example, of avoiding the risk of catheter damage to left main stents which deployed partially outside the vessel, protruding into the aorta)35. Multislice CT imaging could probably be applied to vessels of smaller size in the near future. Awareness of the desirability of noninvasive imaging of coronary stents with MSCT could lead to technological developments, both in MSCT and in stent designs, addressing that goal. Intravascular ultrasound may be used to investigate underlying substrates or mechanisms which might have contributed to the development of restenosis, such as stent under-expansion, collapse or fracture, or the presence of calcific plaque, and that can prove important in a redo procedure15,31,32. But besides, coverage of side-branches, assessment of luminal size and other aspects of interest can be optimally assessed with this technique or with more recently available optical coherence tomography probes.
Assessment of myocardial ischaemia and viability is another key issue in planning secondary revascularisation. Since the sensitivity and specificity of ECG exercise testing decreases significantly after coronary interventions, the use of functional imaging techniques, such as exercise or stress scintigraphy or echocardiography, capable of both detecting and locating myocardial ischaemia, has been recommended10. Magnetic resonance imaging, which allows differentiation of viable myocardium from scar areas, may prove of particular use in these patients. A more selective functional assessment of stents, surgical conduits and native vessels can be performed using pressure guidewire measurements with a spatial resolution not achievable by noninvasive means. The results of a recent RCT, in which a tailored approach to multivessel revascularisation based on individual stenosis assessment with fractional flow reserve, proved to be superior to angiography-guided revascularisation36) suggests that this approach might be particularly useful in secondary revascularisation.
A word on secondary prevention
Secondary prevention constitutes a key element in the healthcare management of cardiovascular patients. Although recent cardiovascular prevention guidelines have placed less emphasis in differentiating “primary” and “secondary” prevention on the grounds that cardiovascular risk is a continuum37, it must be stressed that patients requiring multiple interventions constitute a subset with particular importance within this group, both for the perspective of diminishing acute cardiac events, but also for the prevention of future repeat revascularisation. Previous studies aimed to decrease repeated revascularisation with statins after stent implantation demonstrated a marked benefit in the subgroup of patients studied in terms of major cardiac event reduction38. The explanation rests again on the high-risk profile described above in this article characterised by extensive and chronic atherosclerotic disease. But, besides, secondary prevention may decrease atheromatous progression in native vessels and surgical grafts which, as discussed previously, increases both the rate and difficulty of repeated procedures, and therefore influence outcome.
While this issue deserves a more detailed discussion, its importance must be placed against the background of suboptimal secondary prevention in Europe recently reported in the Euroaspire I, II and III registries39, and of recent data from individual series40. Again, the chances of optimal cardiovascular prevention in these patients will increase if coronary revascularisation is envisaged as a holistic care process, not limited by the short-sighted approach of diagnosis-followed-by-intervention or so-called diagnostic-therapeutic cascade, but contemplated, instead, as an integral part of the cardiovascular biography of patients.

