CASE SUMMARY
BACKGROUND: An 81-year-old man presented with a non-cardiac illness 13 years after quadruple coronary artery bypass graft surgery. Investigations demonstrated an enlarging giant saphenous vein graft aneurysm.
INVESTIGATION: CT thorax, abdomen and pelvis, cardiac CT and coronary angiography.
DIAGNOSIS: Enlarging saphenous vein bypass graft aneurysm.
MANAGEMENT: Transcatheter occlusion of the vein graft aneurysm using an Amplatzer vascular plug.
KEYWORDS: coronary artery bypass graft, saphenous vein graft aneurysm, transcatheter occlusion
PRESENTATION OF THE CASE
A 68-year-old male patient with symptomatic multivessel coronary disease and mild aortic valve disease underwent coronary bypass surgery. At operation a left internal mammary artery graft was placed on the left anterior descending artery (LAD) and saphenous vein grafts (SVG) were placed on the first obtuse marginal, first diagonal and distal right coronary arteries (RCA). At the age of 74 he represented with severe symptomatic aortic stenosis. Coronary angiography showed patent grafts but the body of the SVG to the diagonal artery contained a segment of dilatation measuring 9.7 mm in diameter (Figure 1, Moving image 1). The patient underwent an uncomplicated mechanical aortic valve replacement and was anticoagulated with warfarin.
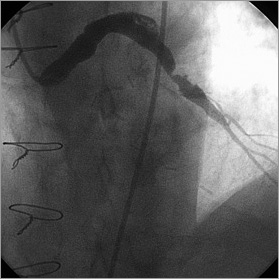
Figure 1. Angiogram prior to aortic valve replacement showing a segment of dilatation and more distal disease in the SVG to diagonal artery (Moving image 1).
At the age of 81 he presented with a septicaemia secondary to a diverticular abscess. During investigation a computed tomography scan of the thorax incidentally demonstrated an aneurysm of the SVG to the diagonal artery, which contained thrombus and measured a maximum dimension of 52 mm and minimum dimension of 37 mm (Figure 2A). A repeat cardiac multiple gated acquisition computed tomogram three months later showed an increase in the size of the aneurysm to 58 mm by 41 mm (Figure 2B). As the patient was asymptomatic the aneurysm was managed conservatively, but a repeat scan (computed tomography coronary angiogram) two years later showed progressive enlargement of the aneurysm to 67 mm by 55 mm (Figure 2C).
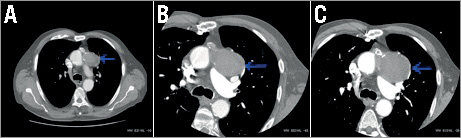
Figure 2. A) CT thorax showing incidental aneurysm of the SVG to the diagonal artery which measured 52 mm by 37 mm. B) Repeat cardiac computed tomogram three months later showing progression in size to 58 mm by 41 mm. C) CT angiogram two years later showing progressive enlargement of the aneurysm to 67 mm by 55 mm.
Coronary angiography showed patent grafts to the LAD and distal RCA but the graft to the marginal artery had occluded. The SVG to the diagonal artery supplied a large graft aneurysm measuring 75 mm by 85 mm in diameter. There was no evidence of flow beyond the aneurysm on multiple contrast injections. The management of the patient was discussed by our multidisciplinary team. The aneurysm was considered to be at high risk of rupture and several therapeutic options were considered:
1. continued conservative care;
2. repeat coronary artery bypass surgery;
3. percutaneous coil embolisation of the graft;
4. transcatheter occlusion of the graft with an Amplatzer vascular plug.
How would I treat?
THE INVITED EXPERT’S OPINION
Reading this interesting case, my first comment is: why did the surgeons not consider replacing the saphenous vein graft (SVG) at the time of surgical valve replacement? The SVG was already diffusely and severely diseased, as evident in Figure 1. Its replacement during the re-do operation would have been appropriate. However, it may not have been performed because of the lack of additional vein conduits, as four SVG had already been used during the first coronary artery bypass graft surgery.
Coming to the current disease status of the patient (the enlarging aneurysm, at high risk of rupture), the patient must currently be 83 years old at least, is asymptomatic and has a diagnosis of slowly but regularly growing aneurysm of an SVG. In the light of these considerations, I would definitely avoid surgery, as this would be his third operation with all the known related risks. I would seriously consider the option of conservative treatment, as the patient remains asymptomatic and is already 83 years old. Percutaneous options to close the aneurysm are attractive; however, their feasibility strongly depends on accurate imaging before and during the procedure. By reviewing the angiographic image of the aneurysm, there appears to be a significantly long “neck” linking the ascending aorta to the aneurysm. This would allow deployment of an adequately sized vascular plug, which should be kept in place by the aortic pressure on the aortic side. Coil embolisation of the aneurysm seems in my view a less attractive option due to the large dimension of the aneurysm itself and to the potential need for a large number of coils to obtain adequate thrombosis of the aneurysm itself. Coiling only the bypass conduit before the aneurysm also appears technically challenging, and thus in my opinion is something to avoid.
One important technical aspect for percutaneous closure of this SVG aneurysm is the assessment of collaterals to the recipient native artery (the diagonal). No flow is visible through the aneurysmatic SVG to the diagonal; however, the authors do not mention the presence of collaterals to the same diagonal. Possible persistence of contrast in the large aneurysm itself may limit the opacification of the distal vessel. The chance of causing a periprocedural infarction by closing the SVG to the diagonal should be considered in this case if collaterals to the diagonal are not visible. This issue should be taken into account in the decision-making process as an additional risk of the procedure.
Thus, in conclusion, given the age of the patient, his stable asymptomatic status and the slow-growing nature of this SVG aneurysm, I would probably consider conservative treatment. A percutaneous option with transcatheter closure of the aneurysm by means of a vascular plug may be an option, given its technical feasibility “a priori”. I would definitely discuss both options (conservative treatment vs. percutaneous closure) with the patient and his family. I would consider carefully the pros and cons of both strategies, and I would probably leave the final decision to the patient himself.
Conflict of interest statement
The author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERT’S OPINION
Giant saphenous vein graft (SVG) aneurysms are a late complication after CABG and usually become evident >10 years after surgery. The exact prevalence and incidence are unclear due to the mostly asymptomatic character of these aneurysms. In the literature, rates of less than 1% are described but systematic analyses are lacking. Occasionally, these aneurysms become symptomatic with new onset of angina, atypical chest pain or myocardial infarction1. Pathophysiologically, this can be explained by the high rate of partially thrombosed venous graft aneurysms, often associated with stenosis directly distal of the vein graft aneurysm and with progressive growth of the aneurysm leading to compression of adjacent structures like the right atrium and/or ventricle1-3. SVG aneurysms with diameters greater than 100 mm have been described1,3.
Ali et al describe the case of a progressively enlarging SVG to the first diagonal coronary artery over a timeframe of 15 years. Already six years after CABG, the SVG was severely degenerated with relevant vessel wall-adherent thrombus, a dilatation of the SVG to 9.7 mm and severe stenosis. At that time, the patient underwent aortic valve replacement that was (for unknown reasons) not combined with a new graft to the diagonal artery. In the subsequent follow-up, the aneurysm increased in size with an estimated annual increase in size of 7.5×9 mm in the last two years, reaching a total diameter of 67×55 mm at 15 years after surgery. The angiogram at this time shows a giant SVG aneurysm with no evidence of flow beyond the aneurysm that was considered to be at high risk of rupture.
The decision-making process in this case is mainly influenced by two questions:
1) what is the true risk of rupture; and
2) what is the appropriate therapy if the risk of rupture is significantly increased?
A couple of case reports have described the rupture of giant SVG aneurysms4-6. The sizes of the aneurysms range from 50 to 110 mm with no systematic recording of information of annual growth rates. In contrast, various reports describe aneurysms of up to 120 mm without any signs of rupture1,3. Taken together, the currently available literature does not allow for an objective risk assessment of SVG aneurysm rupture.
If no reliable data are available, patient and physician have to stick to the best anticipated treatment.
Conservative treatment is a reasonable option in this patient. However, a wait-and-see strategy often leads to an insecure feeling in both the physician and the patient. Re-do surgery is an alternative1, with half of the patients being operated on due to the attendant risk of rupture in a recently published series of 16 patients with SVG aneurysm. In the current case, re-do surgery for the third time is not a preferred option in an 83-year-old gentleman. Other options include coiling7 and occlusion of the graft using Amplatzer plugs. Due to the specific anatomical configuration of the aneurysm it is unlikely that coiling can effectively close the aneurysm. In previous experience7, a significant number of coils are necessary to induce occlusion of the aneurysm, and this largely depends on the size of the perfused lumen. Therefore, in this patient I would vote for the occlusion using an Amplatzer plug. This strategy has previously been used successfully in a giant aneurysm8 and, if carefully performed, should not increase the risk of periprocedural rupture of the aneurysm. However, this option is experimental and no data are available favouring this option over conservative treatment or surgery. Finally, a fifth option may be the injection of thrombin with the protection of an occluded balloon in the neck of the aneurysm to prevent distal embolisation. This option may be hampered by re-opening of the initially thrombosed aneurysm as seen with thrombin injection in femoral pseudoaneurysms.
In any case, a thorough discussion with the patient about available data, therapeutic options, and risks is a prerequisite for the successful treatment of this giant SVG aneurysm. The implantation of an Amplatzer plug would be my primary advice followed by the thrombin injection if not successful.
Conflict of interest statement
The author has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Continued conservative care was not considered appropriate because of the likelihood of progressive enlargement and rupture of the aneurysm. A third cardiac operation was also considered unattractive because of the anticipated high perioperative risk. The multidisciplinary team agreed that the patient should be offered transcatheter occlusion of the graft aneurysm, but the neck of the graft was considered to be relatively large for coil occlusion. He was therefore offered percutaneous occlusion of the graft with an Amplatzer Vascular Plug II (St. Jude Medical, North Plymouth, MN, USA).
After obtaining informed consent, the procedure was carried out via a right femoral arterial approach. A 6 Fr multipurpose guide catheter (Mach 1; Boston Scientific, Galway, Ireland) was positioned in the ostium of the diagonal SVG. Quantitative coronary angiography estimated the diameter of the proximal part of the graft to range from 5 mm to 9 mm, beyond which the graft supplied a giant SVG aneurysm (Figure 3, Moving image 2). A coronary guidewire (Choice PT Extra Support; Boston Scientific, Maple Grove, MN, USA) was passed into the aneurysm and the multipurpose catheter was advanced over the wire into the graft, but provided insufficient support for delivery of an Amplatzer Vascular Plug. An 8 Fr Amplatz Left 1 guide catheter (Boston Scientific, Galway, Ireland) was therefore advanced to the aorta and engaged in the ostium of the diagonal SVG. A 0.025 inch guidewire was then positioned in the aneurysm and a 5 Fr multipurpose catheter (Heartrail ST01; Terumo, Tokyo, Japan) was advanced over the wire and through the 8 Fr guide catheter to the origin of the aneurysm (Figure 4, Moving image 3). A 12 mm Amplatzer Vascular Plug II (St. Jude Medical) was then advanced to the mouth of the aneurysm and deployed by gradual withdrawal of the 5 Fr catheter (Figure 5, Moving image 4). Angiography immediately after deployment of the device showed partial occlusion of the graft (Figure 6, Moving image 5). A repeat coronary arteriogram via the right radial artery on the following day demonstrated complete occlusion of the graft with no residual flow into the aneurysm (Figure 7, Moving image 6). There were no complications and a cardiac computed tomogram six months after the procedure demonstrated shrinkage of the aneurysm to 19 mm by 29 mm diameter (Figure 8).
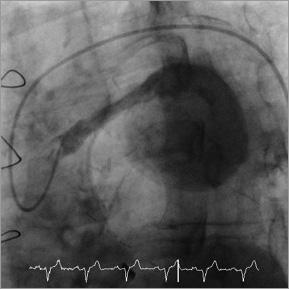
Figure 3. Angiogram of the SVG to the diagonal artery demonstrating a giant SVG aneurysm (Moving image 2).
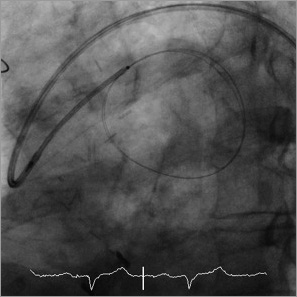
Figure 4. Angiogram showing the 8 Fr Amplatz Left 1 guide catheter engaged in the SVG ostium with a 0.025 inch wire positioned in the aneurysm followed by placement of a 5 Fr catheter over the guidewire.
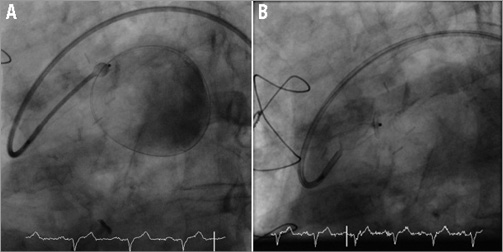
Figure 5. Angiogram showing gradual withdrawal of the 5 Fr catheter to partially deploy (A) and fully deploy (B) the vascular plug within the body of the SVG (Moving image 4).
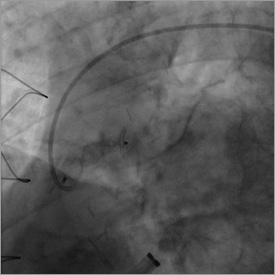
Figure 6. Full deployment of the Amplatzer Vascular Plug at the end of the procedure.
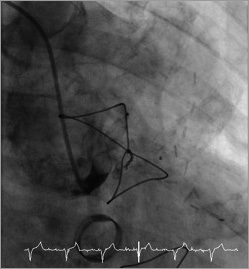
Figure 7. Angiogram one day after the procedure showing complete occlusion of the graft (Moving image 6).
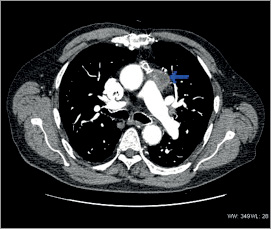
Figure 8. Cardiac CT six months post procedure showing shrinkage of the aneurysm.
Discussion
Aneurysms of coronary SVG are a rare complication of coronary bypass surgery. They usually occur several years after operation but the first reported case occurred six months following surgery9. The true incidence is unknown and difficult to ascertain, as the initial presentation may be sudden death due to aneurysm rupture.
A literature review by Kalimi et al identified 50 cases of SVG aneurysm, of which 45% were asymptomatic and found incidentally during investigations for non-cardiac pathology10. Patients may also present with chest pain, breathlessness and haemoptysis10. Other complications of SVG aneurysm include thrombotic occlusion of the graft11 or coronary thromboembolism12 causing acute coronary syndrome, or compression of neighbouring structures (including other bypass grafts13, right atrium10,15, superior vena cava10, right ventricle14, aorta14, left atrium16, and left pulmonary artery15,16).
Rupture of SVG aneurysms is a rare but potentially fatal complication. Satsu et al reported a SVG aneurysm rupture causing haemopericardium and leading to right-sided cardiac failure16. Rupture into the right atrium17, right ventricle17 and bronchus20 have also been reported.
Cardiac computed tomography was used in our patient and in other case reports to establish the diagnosis, define the anatomy of the aneurysm, and document enlargement of the aneurysm over time. Coronary angiography was required to assess flow beyond the aneurysm and patency of the other coronary grafts. Other imaging modalities that have been used to demonstrate SVG aneurysm include magnetic resonance imaging18,19 and transoesophageal echocardiography19.
The optimal management of SVG aneurysms has not been defined. A retrospective case series compared surgical (two cases) and conservative (11 cases) management and recommended intervention for patients with expanding aneurysms to prevent rupture or where the graft supplies a large territory of myocardium20. Several percutaneous treatment options for saphenous vein graft aneurysms have also been reported. Parry et al described coil embolisation for an enlarging saphenous vein graft aneurysm to the LAD21. Other percutaneous methods include the use of stent grafts and vein-covered stents to exclude smaller aneurysms (diameter around 3 cm)22. A recent case report described successful percutaneous injection of ethylene-vinyl alcohol copolymer to occlude a giant (5.1 cm by 8 cm) expanding saphenous vein graft aneurysm23.
Two previous case reports describe successful use of the Amplatzer Vascular Plug to occlude giant SVG aneurysms24,25. The vascular plug should be around 20-30% larger than the vessel to be occluded, and in our case careful assessment of vascular dimensions by quantitative coronary angiography facilitated selection of an appropriate device24. A unique feature of our case is that we documented progressive enlargement of the aneurysm on serial computed tomography scans before percutaneous intervention, but successful occlusion of the SVG resulted in regression of the aneurysm. We speculate that percutaneous occlusion of the SVG has altered the natural history of the aneurysm and abolished the risk of rupture.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Angiogram prior to aortic valve replacement showing a segment of dilatation and more distal disease in the SVG to diagonal artery.
Moving image 2. Angiogram of the SVG to the diagonal artery demonstrating a giant SVG aneurysm.
Moving image 3. Angiogram showing the 8 Fr Amplatz Left 1 guide catheter engaged in the SVG ostium with a 0.025 inch wire positioned in the aneurysm followed by placement of a 5 Fr catheter over the guidewire.
Moving image 4. Angiogram showing gradual withdrawal of the 5 Fr catheter to deploy the vascular plug within the body of the SVG.
Moving image 5. Full deployment of the Amplatzer Vascular Plug at the end of the procedure.
Moving image 6. Angiogram one day after the procedure showing complete occlusion of the graft.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Angiogram prior to aortic valve replacement showing a segment of dilatation and more distal disease in the SVG to diagonal artery.
Moving image 2. Angiogram of the SVG to the diagonal artery demonstrating a giant SVG aneurysm.
Moving image 3. Angiogram showing the 8 Fr Amplatz Left 1 guide catheter engaged in the SVG ostium with a 0.025 inch wire positioned in the aneurysm followed by placement of a 5 Fr catheter over the guidewire.
Moving image 4. Angiogram showing gradual withdrawal of the 5 Fr catheter to deploy the vascular plug within the body of the SVG.
Moving image 5. Full deployment of the Amplatzer Vascular Plug at the end of the procedure.
Moving image 6. Angiogram one day after the procedure showing complete occlusion of the graft.

