
Abstract
Covered stents offer an effective bail-out strategy in vessel perforations, are an alternative to surgery for the exclusion of coronary aneurysms, and have a potential role in the treatment of friable embolisation-prone plaques. The aim of this manuscript is to offer an overview of currently available platforms and to report results obtained in prior studies.
Introduction
Metallic stent platforms covered with a synthetic or biological membrane were originally developed in an effort to address the limitations of stenting, such as thrombosis and restenosis, dividing the thrombogenic struts from the bloodstream and preventing smooth muscle cell migration causing neointimal hyperplasia. Unfortunately, neither of these two hypotheses passed the scrutiny of randomised clinical trials. Nevertheless, covered stents remain essential in catheter laboratories, since they are utilised in a number of niche situations, mainly for emergency treatment of coronary perforations. This manuscript aims to offer an overview of currently available platforms and to report results obtained.
AUTOLOGOUS VEIN/ARTERY COVERED STENTS
These stents marked the start of the use of covered stents in coronaries but now remain important only from a historical point of view. The preparation process included isolation of a length of vessel, which was then passed into the stent and sewn at both ends, in the middle of the outer part of the stent, so that both the luminal and external surfaces were covered by the graft. These stents were fundamentally used to improve the stent biocompatibility, accelerating endothelialisation and reducing the proliferative response of the artery wall1. Indeed, early trials in swine iliac arteries demonstrated the feasibility, safety as well as low thrombosis rates of both artery- and vein-covered stents1,2. Clinical applications showed that vein-covered stents could be safely prepared and implanted during angioplasty but there was no obvious benefit in restenosis, target vessel revascularisation or event-free survival when compared to bare metal stents3. In addition to the long and demanding preparation process, with surgical exposure of a vessel in the forearm, these stents were very bulky due to their two-layer structure and were poorly deliverable. Still, they represented an important proof of concept prototype that paved the way for the introduction of commercial ready-made covered stents.
POLYTETRAFLUOROETHYLENE-COVERED STENTS
Polytetrafluoroethylene (PTFE) is a synthetic fluorocarbon polymer with a wide range of applications in medicine, including manufacturing of vascular grafts in patients with or without poor autologous conduits. By far the most frequently used stent in this group is the GRAFTMASTER® (previously JoGraft; Jomed, and now GRAFTMASTER; Abbott Vascular, Santa Clara, CA, USA), which is constructed using a sandwich technique, whereby a layer of ePTFE is placed between two 316L stainless steel stents (Table 1). A similar technology is applied in the Direct-Stent® (InSitu Technologies Inc., St. Paul, MN, USA), while the BeGraft coronary stent graft system (Bentley Innomed GmbH, Hechingen, Germany) combines a single layer cobalt-chromium stent platform with an ePTFE membrane clamped at the stent ends.
POLYURETHANE-COVERED STENTS
Polyurethanes are known for their good biocompatibility together with their good physiochemical and mechanical properties, and are widely used in cardiovascular applications, such as heart valves and pacemaker leads. While polyurethane-covered stents for peripheral use have been used for decades, a dedicated coronary device was only recently released (PK Papyrus; Biotronik, Bülach, Switzerland) based on their workhorse platform, covered by a 90 µ thick polymer using electrospinning technology that yields a thinner and relatively flexible material.
PERICARDIUM-COVERED STENTS
The pericardium is a collagen-rich biological tissue and is broadly applied as a biomaterial for tissue engineering. The currently available Aneugraft® Dx stent (ITGI Medical, Or Akiva, Israel) consists of a flexible 316L stainless steel balloon-expandable stent, which is covered with a 105 µ thick single layer of equine pericardium.
Uses of covered stents
CORONARY PERFORATIONS
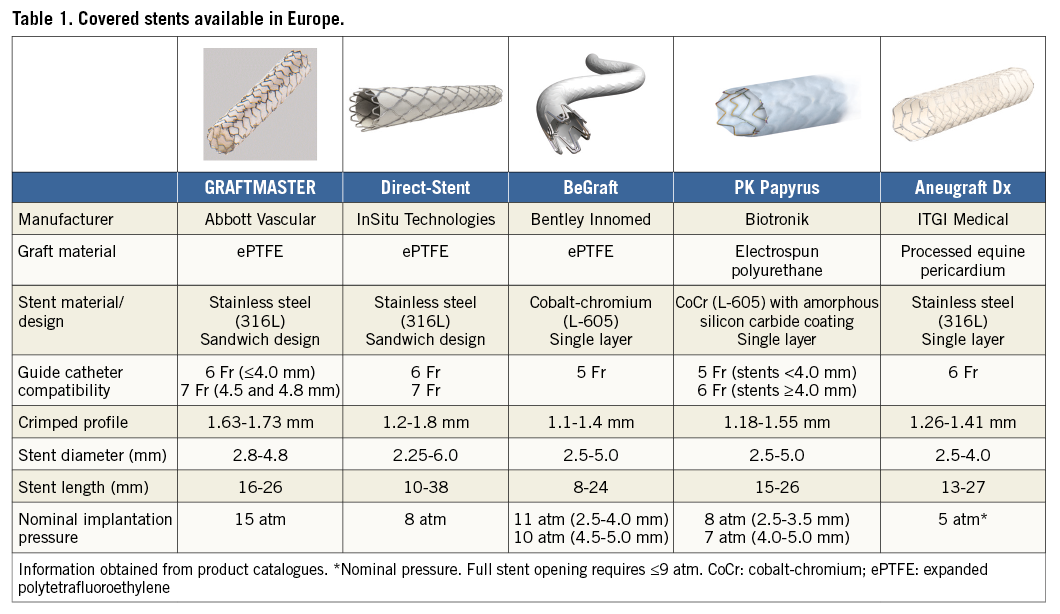
Coronary artery perforation is a rare but potentially disastrous complication of percutaneous coronary interventions (PCI). Management strategy is based on the severity and characteristics of bleeding. Ellis et al4 classified coronary perforation into three types: Type I, extraluminal crater without extravasation; Type II, pericardial or myocardial blushing; Type III, perforation ≥1 mm diameter with contrast streaming; and cavity spilling. While a conservative approach can be attempted for the least severe perforations, implantation of covered stents, coil embolisation, and surgery are needed for brisk ruptures5. In Type II-III perforations of the proximal or mid part of a coronary artery, covered stents allow rapid sealing of the coronary leak and are preferred as second line treatment if haemostasis cannot be achieved by prolonged balloon inflation and reversal of anticoagulation (Figure 1).
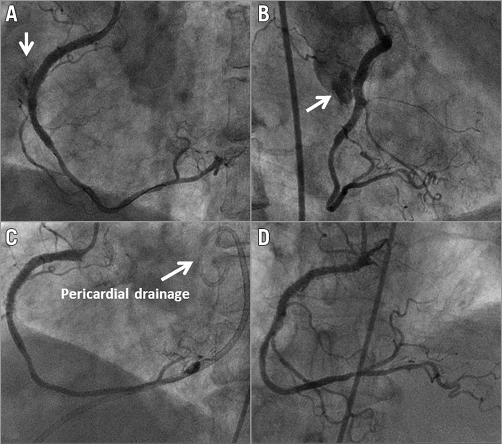
Figure 1. Covered stents for the treatment of a perforation. Coronary artery perforation after deployment of two everolimus-eluting stents in the right coronary artery (A & B). A 3.0×16 mm PTFE-covered stent (GRAFTMASTER) was immediately deployed in these stents, completely sealing the perforation (C). Follow-up angiography at 15 months showed the patency of the stents (D).
There is ample literature on the use of covered stents for both native coronary artery and bypass graft perforations6-9, but these articles are often case reports or small retrospective observational studies, since randomisation would be impossible in this bail-out situation. Briguori et al compared the efficacy of the PTFE-covered stents for sealing of coronary perforations with that of non-covered stents used prior to the availability of PTFE-covered stents at their institution, and showed a high success rate and a favourable early outcome with covered stents10. In contrast, one retrospective study found that the use of covered stents to manage perforations was not associated with any reduction in adverse outcomes, such as the development of cardiac tamponade, need for emergency coronary artery bypass grafting, or in-hospital death. However, these results were flawed by the difference in the perforation grade between the groups11. Al-Lamee et al reported the management of 56 patients with grade III coronary perforation in two high-volume centres over 16 years. Covered stents were deployed in 46.4% of patients (n=26) resulting in a high success rate with haemostasis achieved in 84.6% of cases (n=22), almost exclusively using PTFE-covered stents12. A recent report of nine cases of perforations for which balloon dilatation was not sufficient showed the success of the use of pericardium-covered stents even in cases with complex coronary anatomy13.
PRACTICAL TIPS
An interventional cardiologist must be primed for this complication, but the scarcity of these events, especially grade III perforations, limits the ability to acquire and maintain the necessary skills in managing this complication. When using oversized balloons, or balloons at very high pressure or in resistant lesions with dog-boning, it is good practice to perform a contrast injection after dilatation while the balloon is still within the guide catheter. In this way a perforation is recognised early and can be sealed by advancing the same balloon back across the vessel segment. The priority is obviously to maintain haemodynamics, and brisk ruptures with immediate tamponade require immediate balloon dilatation to interrupt coronary flow and gain time to perform pericardiocentesis. For ruptures not healing and causing a rapid development of tamponade, a second guide catheter should be considered, releasing the balloon only to pass a new wire and the covered stent14. Caution should be taken to remove the first guide catheter slightly from the coronary ostium, whilst maintaining the sealing balloon inflated, before the engagement of the second catheter15.
Despite the decreased crossing profiles of the newer covered stents, these devices are still bulky and have lower deliverability compared to the non-covered stents. Therefore, one must be prepared to face difficulties in delivering these stents, especially in tortuous and calcified vessels. Stiffer wires or buddy wires are a good option when the delivery of the covered stent is a planned procedure but might be difficult in an emergency setting. Anchoring strategies or guiding catheter extensions are the alternatives; however, compatibility issues should be considered (a 6 Fr GuideLiner® [Vascular Solutions, Inc., Minneapolis, MN, USA] is in fact equivalent to 5 Fr). Second, these stents might require higher pressure to ensure adequate stent expansion (GRAFTMASTER) or extreme attention in order not to exceed the pressure compliance of the balloon, overexpanding it and causing tears in the membrane (pericardium-covered stents). Therefore, sizing the covered stent 1:1 is essential as both overexpansion and underexpansion are not desirable. Covered stents offer more resistance to balloon expansion and, during inflation, a more prominent “dog-bone” effect is observed than with conventional stents16. If the balloon inflation is performed slowly, this facilitates trapping of friable material prone to embolisation, such as thrombus or superficial lipid plaques. During inflation, the membrane can shorten, which should be kept in mind for overlapping or adequate coverage of the perforation site17. After stent deployment and once the patient is haemodynamically stable, intracoronary imaging techniques may be useful to ensure correct stent expansion. After adequate sealing of the perforation, dual antiplatelet therapy (DAPT) should be started as soon as possible. In the absence of evidence regarding the duration of antiplatelet therapy following covered stent implantation, DAPT should be continued long term, unless otherwise contraindicated.
EXCLUSION OF CORONARY ANEURYSMS AND FISTULAS
Coronary artery aneurysms (CAA) are relatively uncommon and are usually identified incidentally during angiography.
The indication for treatment and the best treatment modality still need to be defined. If a decision is made to exclude the aneurysm, covered stents can be safely used in native arteries (Figure 2) or grafts, including DES-related CAA in patients with a suitable anatomy, as an alternative to surgery18,19.
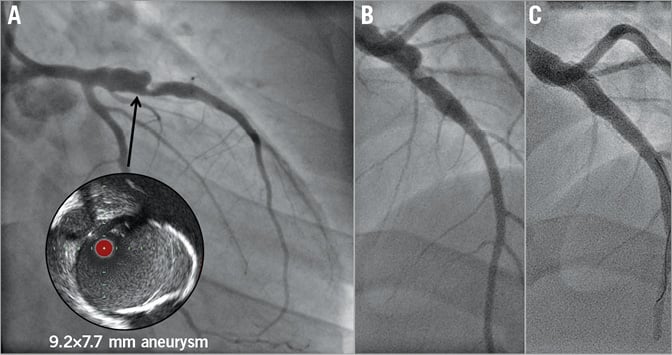
Figure 2. Giant coronary aneurysms post Kawasaki disease in the left anterior descending artery (LAD) of a 25-year-old male. A) Intravascular ultrasound view of the aneurysm (circle). B) Different coronary projection. C) Final angiography demonstrating exclusion of the aneurysm with two covered stents (5.0×26 and 4.0×15 mm PK Papyrus). Modified from Di Mario C et al45, with permission.
In a previous case series of seven CAA patients treated with PTFE-covered stents, angiographic follow-up at 10±6 months showed good results with restenosis occurring in only one patient, necessitating a repeat PCI18. Moreover, at 35±8 months clinical follow-up, six patients were symptom-free. In an analysis of the reported cases including the abovementioned series, Szalat et al reported that only five of the 24 patients who received stents were found to have restenosis on follow-up angiography, and these patients had larger aneurysms (>10 mm in diameter)20. They also compared these data with an earlier report reviewing the cases having operative therapy for CAA21: they suggested that covered stents might be a good option for relatively smaller CAA (5.8-10 mm in diameter) and that surgery might be preferable for larger ones (above 10 mm)20. However, this was not a controlled study; moreover, the number of patients was too small and the data were heterogeneous. Therefore, these recommendations can only be considered as speculative.
Coronary artery fistulas are another rare anomaly, and are defined as abnormal communications between the coronary artery and a cardiac chamber or another low-pressure vessel (for instance, the pulmonary artery), bypassing the myocardial capillary bed. Coronary fistulas can be treated percutaneously with a number of options, and most frequently with coil embolisation. Covered stents can offer an easier solution and create a mechanical barrier between the fistula and the feeding vessel, but the coronary artery where the fistula originates has often grown too large to be approachable, and there is concern about exposing an otherwise normal artery to the complications associated with stent implantation. A selective niche utilisation in the presence of a concomitant stenosis22 or aneurysms23, or when the anatomy is not suitable for coil embolisation (e.g., presence of multiple branches)24, would be more plausible.
PRACTICAL TIPS
Occasionally, the length of the CAA can exceed the available length of a covered stent, so that two covered stents must be used in a sequential manner. Normally, the distal stent is deployed first but, when the stent remains unsupported in the middle of the aneurysm, the more proximal stent may have difficulty to advance and may distort the distal stent. Alternatively, a technique from proximal to distal sequential PTFE-covered stent implantation may be considered25.
SAPHENOUS VEIN GRAFT INTERVENTIONS
Saphenous vein graft (SVG) interventions remain technically challenging and are associated with higher rates of periprocedural myocardial infarction. This is largely due to the friable, degenerated atheromatous and thrombotic debris that embolises distally, causing microvascular obstruction and vasospasm26. Although embolic protection devices (EPD) successfully prevent the thromboembolic complications associated with PCI to SVGs, distal lesions are not anatomically suitable for filter or distal occlusion balloons. Moreover, the particles protruding through the stent struts may continue to shed debris even after the PCI is completed and the EPD is retrieved27. Therefore, the concept of using a covered stent to trap all potentially embolic debris against the vessel wall at the treatment site is appealing (Figure 3).
Figure 3. Use of covered stents in saphenous vein graft lesions. A) A lesion with high signal attenuation, where (B) indicates a normal segment. After implantation of a pericardium-covered stent, optical coherence tomography confirmed exclusion of the lesion and good strut apposition (C & D). Modified from Tyczynski et al36, with permission.
In an initial registry, PTFE-covered stents appeared to be a safe and efficient treatment strategy for SVG lesions with a low incidence of restenosis and target vessel revascularisation28. These promising results have fuelled a number of randomised trials. However, these trials showed results either similar to or worse than bare metal stents in terms of restenosis or MACE29-31. These results were obtained with the double-layered GRAFTMASTER that required high-pressure inflation, which possibly caused more acute embolisation. When the self-expanding PTFE-covered nitinol Symbiot stent (Boston Scientific, Marlborough, MA, USA) was tested, results were equally poor32,33, leading to the withdrawal of the device.
The main results of randomised trials in the use of covered stents for saphenous vein graft lesions are presented in Figure 4.
The experience with pericardium-covered stents (PCS) in SVGs is limited to two small registries with a limited follow-up. The SLEEVE-I and II studies included 63 patients and 80 lesions and showed high procedural success rates and low MACE rates at 30 days in de novo or restenosed SVG lesions34.
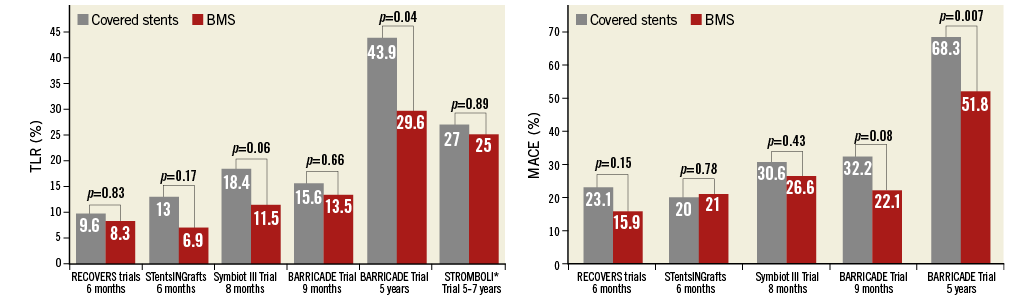
Figure 4. Randomised trials in the use of covered stents for saphenous vein graft lesions. *Comparison between Symbiot™ covered stent and conventional bare metal stent (Express II; Boston Scientific) with or without a FilterWire. MACE: major adverse cardiac events; TLR: target lesion revascularisation
PRACTICAL TIPS
There is also the possibility that the high profile of these stents may lead to significant distal embolisation when crossing the target lesion, and therefore they should not be considered as an alternative to EPD. A previous study by Blackman et al could not find significant difference in distal embolisation between the Symbiot stent and BMS using a FilterWire EZ™ Embolic Protection System (Boston Scientific)35. However, covered stents can be considered as devices to prevent late dislodgement of friable plaques, particularly occurring in the first hours of stenting, for which EPDs are of no value36. There is a clear unmet need in the reconstruction of long occlusion of SVGs, full of thrombotic material. Long low-profile non-thrombogenic covered stents can offer a solution when the approach through the native artery is truly impossible.
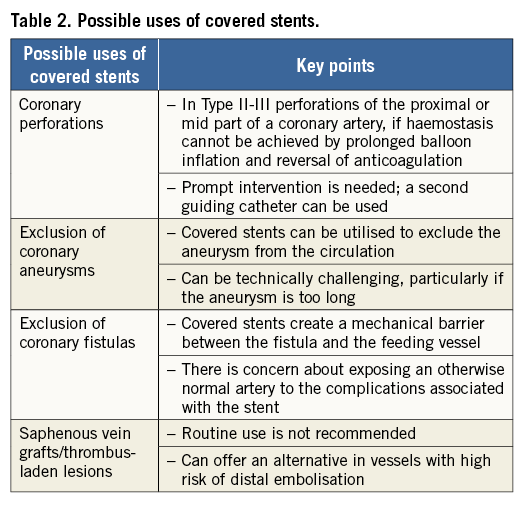
The possible uses of covered stents and the key points are summarised in Table 2.
OTHER USES OF COVERED STENTS
Theoretically, covered stents can be deployed in the left anterior descending artery across the septal artery ostia to obstruct the artery and thus decrease the left ventricular outflow tract gradient as an alternative to alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. Only anecdotal cases of patients reported immediate and long-lasting elimination of the gradient37; however, the dual supply of the septum makes it more likely that the obstruction will persist or return38, and the potential value of this strategy has never been properly demonstrated in a series of patients. Therefore, to date this strategy remains of unproven value.
Covered stents were also utilised in thrombus-laden lesions in native coronary arteries. Gunn et al described the treatment of 10 lesions with the pericardium-covered stent in nine patients presenting with acute coronary syndromes associated with massive thrombus burden, in whom conventional percutaneous and pharmacological methods of dispersal or aspiration were not appropriate or successful39. Investigators reported high immediate procedural success, one patient with acute stent thrombosis, and no additional adverse events occurring in six patients who completed the 12-month follow-up. An alternative to this application can be the fine fabric mesh-covered stent (MGuard™; InspireMD, Tel Aviv, Israel)40.
CONCERNS ABOUT COVERED STENTS
In coronary arteries, covered stents were initially designed as mechanical barriers to prevent in-stent restenosis, but comparisons with bare metal stents showed no advantage or higher rates of in-stent restenosis. Neointimal proliferation is frequent at the edges of the covered stent16,29,30, possibly related to the vessel trauma caused by high-pressure dilatation and lack of full PTFE coverage in the GRAFTMASTER stent29. On the other hand, a study by Papafaklis et al reported promising results in 14 patients with overlapping implantation of covered stents with DES at the edges. An angiographic follow-up at approximately 10 months showed restenosis only in one patient, in a segment with no overlap. At a mean follow-up of 21.9 months, no deaths or myocardial infarctions were reported41. Hou et al investigated a similar concept in nine patients by assessing the PTFE-covered stents with underlying longer sirolimus-eluting stents. At a 9.4 (±3.4)-month angiographic follow-up, there was no stent-edge or stent-segment binary restenosis, while all covered stents had full and smooth neointimal coverage, verified by OCT42.
Thrombosis may also be a concern for the use of covered stents16 (Figure 5). Al-Lamee et al reported an incidence of definite stent thrombosis of 8.6% in 46 patients12. A potential mechanism for thrombogenicity may be the late re-endothelialisation of PTFE-covered stents. Despite conflicting results43, delayed endothelialisation is likely44; however, newer stents may have different properties in terms of endothelialisation. Furthermore, prolonged treatment with the more potent newer anti-aggregants might also have a role in preventing stent thrombosis9. Finally, the physical barrier formed by the covered stent can compromise the side branch flow. Consequently, alternative options should be considered when a major side branch is likely to be occluded. Naturally, in bail-out situations, all of the concerns are offset by the possible gain.
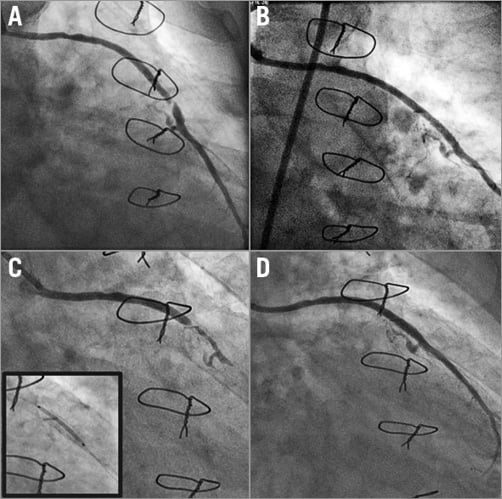
Figure 5. Covered stents in saphenous vein grafts. Occlusive lesion of the saphenous graft (A) was successfully treated with a pericardium-covered stent (B). However, repeat angiography after four months showed total occlusion of the covered stent (C), which was treated with implantation of an everolimus-eluting stent (D).
Conclusion
Covered stents offer an effective bail-out strategy in vessel perforations, are an alternative to surgery for the exclusion of coronary aneurysms, and have a potential role in the treatment of friable embolisation-prone plaques. Novel stents may overcome some of the technical limitations of first-generation devices but greater experience and longer follow-up are imperative before expanding indications outside bail-out or niche anatomical conditions.
Acknowledgements
Ismail Dogu Kilic was supported by a research grant from the “The Scientific and Technological Research Council of Turkey (TUBITAK)”.
Conflict of interest statement
The authors have no conflicts of interest to declare.

