
Abstract
Aims: Covered stents are mostly used for coronary perforations with a high risk of early adverse events; however, their long-term outcome is unknown. The aim of this study was to elucidate the short- and long-term outcome of patients treated with covered stents compared to all other stented patients.
Methods and results: The Swedish national registries from 2005-2017 disclosed 265 patients who had received 366 covered stents. Their outcomes were compared to all other stented patients (197,948) who had received 320,784 stents. Compared to regular stents, covered stents showed significant differences (p<0.001) in the short and long term in relation to in-stent restenosis (ISR), target lesion revascularisation (TLR), re-infarction (MI), re-PCI and mortality, the rates of which were all higher. The higher mortality was concentrated within the first month, as a landmark analysis at that time point, adjusted for age and procedural indication, demonstrated no future difference in mortality (HR 1.02 [0.78-1.33], p=0.877). Stent thrombosis (ST) within one year was reported to be higher in covered stents than in other stents. However, no ST was reported in equine pericardial covered stents.
Conclusions: This observational study including the entire Swedish population shows that patients receiving covered stents have a significantly higher risk of all adverse events. Reassuringly, in the long term, mortality appears to be similar to that in other stented patients.
Abbreviations
ANU: Aneugraft
CABG: coronary artery bypass grafting
FFR: fractional flow reserve
GM: GRAFTMASTER
HR: hazard ratio
ISR: in-stent restenosis
MI: myocardial infarction
OU: Over and Under
PAP: Papyrus
PCI: percutaneous coronary intervention
SCAAR: Swedish Coronary Angiography and Angioplasty Registry
ST: stent thrombosis
SWEDEHEART: Swedish Web-system for Enhancement and Development of Evidence-based care in Heart Disease Evaluated According to Recommended Therapies
TLR: target lesion revascularisation
Introduction
Covered stents are unique in design as they set a barrier between the coronary blood vessel wall and its lumen. They have been examined in several indications.
Coronary artery perforations are feared but are luckily a rare complication, 0.1-0.8%1,2. Perforations are associated with a high incidence of tamponade, myocardial infarction and surgical repair and death. There are several reports showing the superiority of covered stents to seal type III perforations and, in comparison with non-covered stents, for prolonged balloon inflation and heparin reversal1,3-7.
Degenerated saphenous vein grafts are challenging to treat due to the high risk of distal embolisation of friable atherosclerotic material or thrombus and they have a high restenosis rate. Covered stents open in a dog-bone fashion and could potentially capture/seal off the friable atheromatous debris between the abluminal surface of the covered stent and the inner lumen wall. However, randomised trials have shown inferiority of covered stents compared to bare metal stents (BMS) with respect to restenosis and target vessel failure8-11.
Pericardial covered stents have been successfully used in ST-elevation myocardial infarction in a limited number of cases. Due to the dog-bone expansion, thrombus material may be trapped between the covered stent and the artery wall, potentially capable of reducing infarct size12.
The literature provides case reports of successful exclusion of aneurysms, pseudoaneurysms, occlusion of a septal branch, and obliteration of severe thrombus formation or occlusion of a fistula with covered stents13-16. However, the risk and patency of covered stents in such rare indications remains unknown.
Since covered stents are rarely used and mostly in urgent or acute indications, the evidence of their outcome or comparison between different types of covered stent needs to be derived from registries.
In this study, we present data from the national Swedish registry comparing covered stents with the overall stented population for more than a decade and elucidate differences in their behaviour in the long term.
Materials and methods
The Swedish Coronary Angiography and Angioplasty Registry (SCAAR) is an integrated part of the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). SCAAR records on-line, through a web interface, all consecutive patient data from all centres (n=30) performing PCI in Sweden17. The internet-based system provides each centre with immediate and continuous feedback on processes and quality-of-care measures. Monitoring and verification of registry data have been regularly performed in at least 1/3 of the hospitals since 2001 by comparing 20 entered variables in 20 randomly selected interventional procedures per hospital and year with the patients’ hospital records. Automatic quality control is also continuously performed on the SCAAR interface. The recordings of clinical and angiographic data are indicated as complete and the case can be closed only if all the mandatory variables have been inserted (for further description of the database please visit www.ucr.uu.se/en).
If any subsequent angiography is performed, registration of restenosis and stent thrombosis (ST) in every previously implanted stent is mandatory. In-stent restenosis (ISR), as registered in SCAAR, is defined as a stenosis assessed by angiographic visual estimation (>50%) or by fractional flow reserve (FFR) ≤0.80 in a previously stented segment identified by coronary angiography for any clinical indication performed anywhere in the country. In keeping with the Academic Research Consortium18, definite ST is defined as symptoms suggestive of an acute coronary syndrome and angiographic evidence of ST. Occurrence of ST or restenosis in a stent is a censoring event for this stent and analysed either as a restenosis or as a subacute stent thrombosis. Target lesion revascularisation (TLR) is defined as any attempt to perform new PCI in the same coronary segment as the index stenting was done. Information on repeat PCIs (re-PCI) and TLR is taken from SCAAR. Procedural success after PCI treatment of the coronary lesion is defined as residual stenosis <50%, decreased grade of stenosis after intervention by at least 20%, normal blood flow and no serious complication. Perforations among many other adverse events are registered both from the cath lab and in the ward department at discharge by the treating physician. Myocardial infarction (MI) is defined as any rehospitalisation after the index procedure registered in the Swedish Register of Information and Knowledge about Swedish Heart Intensive Care Admissions (SWEDEHEART/CCU) with International Classification of Diseases codes I21 and I22.
Vital data are obtained from the Swedish National Population Registry. The merging of the registries is performed by Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden, and is approved by the local ethics committee at Uppsala University.
Adverse events, including perforation, tamponade, death, ISR, TLR, ST, new MI, coronary artery bypass grafting (CABG), and re-PCI, are summarised during the procedure, at one month and one year after the index procedure and also followed throughout the study period.
STATISTICAL ANALYSIS
Baseline characteristics are summarised with means and standard deviation for continuous variables and percentages for discrete variables. Cumulative event rates were estimated using the Kaplan-Meier method as time to event. Weibull regression was used for calculations of the hazard ratio. All individual covered stent-treated patients were followed until January 2018 or until restenosis or death occurred in the reported segment after implant.
Only covered stents without missing data are presented. Five patients with more than one type of covered stent used in the procedure were the only patients excluded and only at the patient level.
In a landmark analysis, the effect of the covered stent was also evaluated in the time period after the first 30 days. Adjustment was carried out for age and for the indication of the procedure (stable CAD, STEMI, unstable CAD, non-STEMI and other).
Only p-values <0.005 were considered significant. Higher values up to 0.05 were considered hypothesis-generating19.
All analyses were conducted in SPSS, Version 24 (IBM Corp., Armonk, NY, USA) and R, version 3.3.3 equipped with the survival and the SurvRegCensCov packages (www.r-project.org).
THE COVERED STENTS EVALUATED
The GRAFTMASTER® RX (previously named Jograft) coronary stent graft system (GM; Abbott Vascular, Santa Clara, CA, USA) became available in 1998. It is constructed using two stainless steel GRAFTMASTER stents, sandwiching a thin layer of expandable polytetrafluoroethylene (PTFE). During most of the study period the balloon-expandable graft system was only available in sizes from 3-4 mm and was 6-7 Fr guide catheter compatible.
The “Over and Under” (OU) balloon-expandable equine pericardium-covered stainless steel stent (Amnis Therapeutics, Or Akiva, Israel) was marketed from June 2009 to 2012. The covered stent was packaged wet in a plastic tube with glutaraldehyde, from which it needed to be rinsed before usage. It was a stainless steel stent (90-99 µm strut thickness), 100% covered with a heterologous single 105±5 µm layer of equine pericardium.
The OU was replaced in 2012 by the second-generation pericardium-covered Aneugraft stent (ANU; Amnis Therapeutics). It is delivered dry and thus needs no rinsing. The stent and equine pericardial cover remain the same as for the OU. The graft is provided in sizes from 2.5-4 mm and is 6 Fr guide catheter compatible.
The PK Papyrus (PAP; Biotronik, Bülach, Switzerland) is a single layer 90 µm electrospun polyurethane-covered cobalt-chromium stent. It was introduced in 2015 and has the lowest profile compared to the above, is 5-6 Fr guide catheter compatible and available in sizes from 2.5-5.0 mm.
ETHICS APPROVAL
The study was approved by the regional scientific ethics committee of Lund University. Since all patients were anonymised in the study (with their social security number substituted by a unique SWEDEHEART specific ID number), no informed consent was deemed necessary by the scientific ethics committee.
Results
From May 2005 until January 2018, 197,948 patients (27% female) underwent PCI with stent treatment and received 320,784 stents in Sweden. In this period, 265 patients received 366 covered stents. Two hundred and sixty-five patients receiving covered stents were available for at least one-year follow-up. The mean follow-up time for the covered stent patients was 6.2 (SD 3.2) years. In an attempt to elucidate whether there were any important differences between types of covered stent, their data are shown separately. One hundred and forty-two patients were treated with 199 GM, 36 patients with 50 OU, 27 patients with 43 ANU and 60 patients received 74 PAP.
Background and procedural characteristics are shown in Supplementary Table 1. Characteristics of lesions are shown in Supplementary Table 2. Adverse events during the procedure are shown in Table 1. Landmark analysis of adverse events is displayed in Table 2. Developments over time are shown in Figure 1A-Figure 1D and Figure 2A-Figure 2D. Procedural developments over time are shown in Supplementary Figure 1.
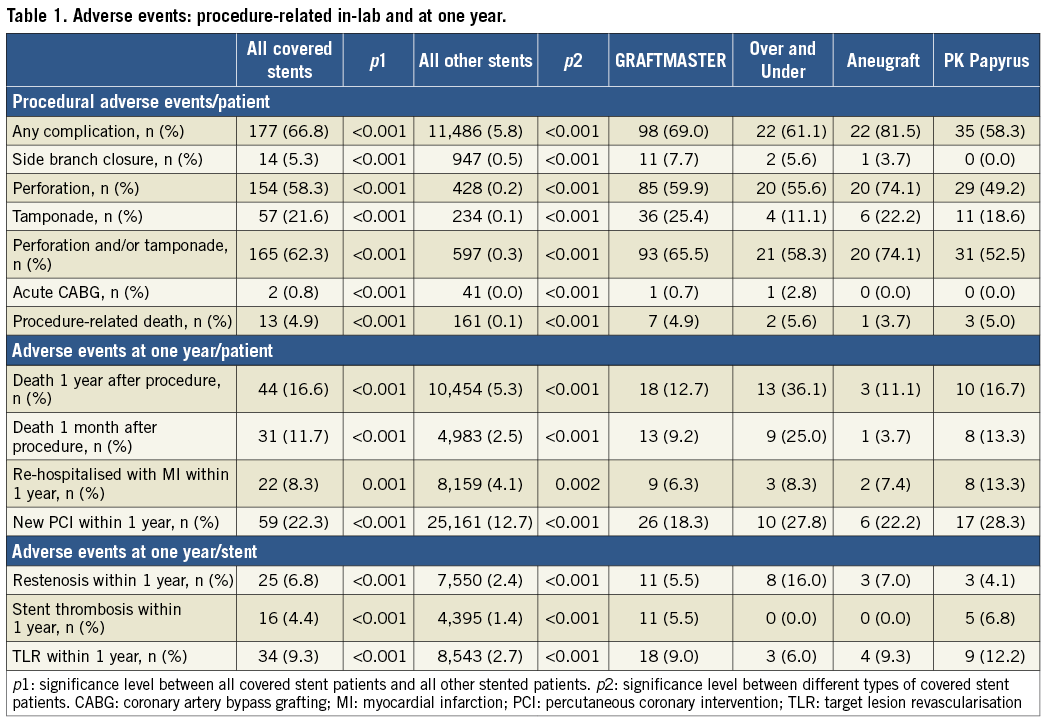
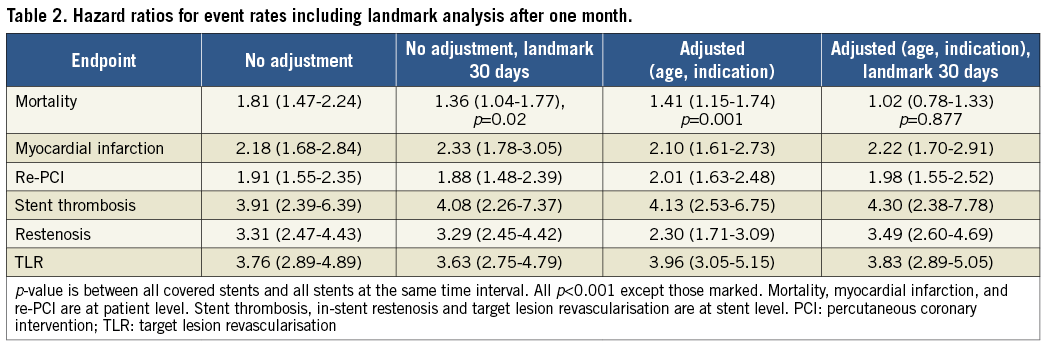
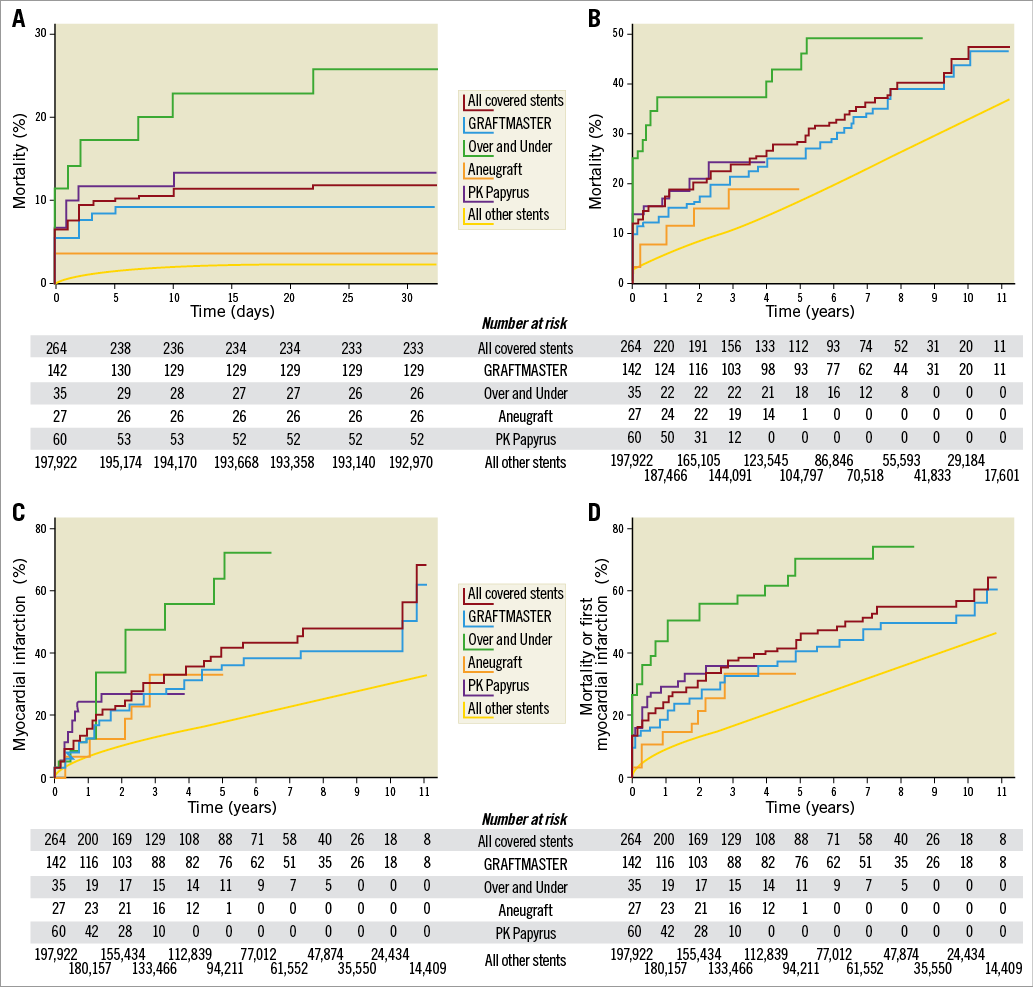
Figure 1. Unadjusted Kaplan-Meier curves for outcomes after covered stents 2005-2017. A) Mortality in the first month in patients with covered stents and other stents implanted. B) Mortality in patients with covered stents and other stents implanted. C) First myocardial infarction after implantation of covered stents and other stents. D) Mortality or first myocardial infarction after implantation of covered stents and other stents.
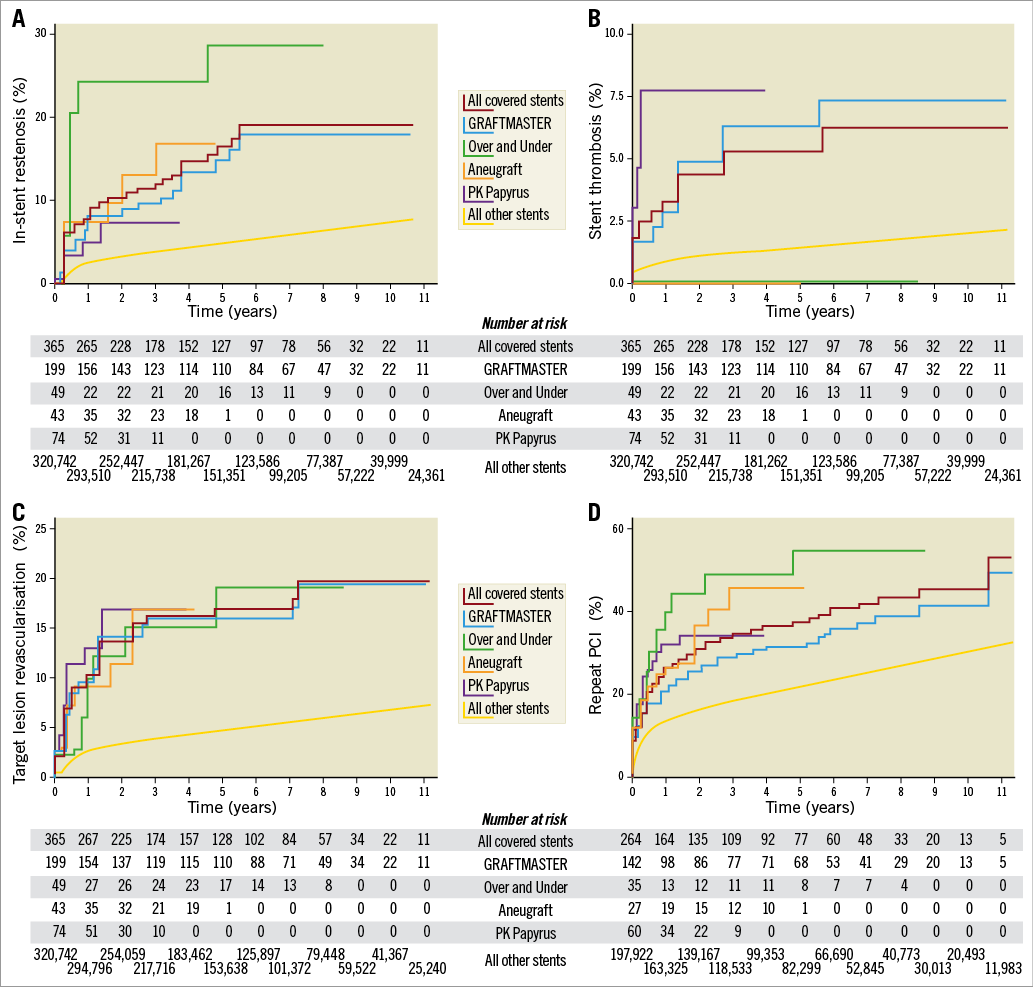
Figure 2. Unadjusted Kaplan-Meier curves for outcomes after covered stents 2005-2017. A) In-stent restenosis in covered stents and other stents implanted. B) Stent thrombosis in covered stents and other stents implanted. C) Target lesion revascularisation in covered stents and other stents implanted. D) Repeat PCI after implantation of covered stents and other stents.
Two hundred and one patients received only one covered stent, 50 had two, 15 had three, two patients received four and one received five covered stents. Finally, one patient had seven covered stents implanted in an aneurysmatic saphenous vein graft, which may serve as an example of the indication “other” (Table 1) and stenosis class “other” (Table 2).
Patients treated with covered stents differed significantly from other stented PCI patients. They were younger, were more often female, had more hypertension, hyperlipidaemia, more previous MI, CABG, and PCI. The treated lesions were also more complex, with significantly higher rates of type B2/C lesions and saphenous grafts. More had three vessel and left main disease and the procedures had a higher use of intravascular ultrasound (IVUS) but similar use of coronary physiology measurements and optical coherence tomography (OCT). The covered stents used were shorter in length and larger in diameter compared to other stented patients.
The operator reported successfully implanted covered stent(s) into the treated segment in 95.3% and an overall successful procedure in 85.7%, with a complete revascularisation rate of 52.9% - all significantly less than for other stented patients (99.0%, 98.3% and 66.2%, respectively).
Procedure-related complications are shown in Table 1. The majority of covered stent patients had more than one complication reported. Importantly, the key findings in these complications were that they were not caused by the covered stents, but rather the reasons for using them. For example, perforation and tamponade were complications of the PCI procedure leading to the use of a covered stent, whereas a side branch closure was probably caused by the use of a covered stent. Emergency CABG was only seen in two patients.
While the majority of covered stents were used for perforations and/or tamponades in 165 patients, in 100 patients they were used for other indications, such as thrombus or aneurysm exclusion.
Patient death during the procedure, at one month and one year, readmission for a new MI within the first year after index procedure, and re-PCI are shown in Table 1. They were all significantly higher for covered stent patients compared to other stented PCI patients.
Covered stent ISR, ST, and TLR rates at one year are reported in Table 1. They were all significantly higher for covered stents compared to other stents.
Compared to other stented PCI patients, hazard ratios for adverse events in a covered stent with and without a landmark analysis are shown in Table 2. Crude mortality was higher (p<0.02) at one year. Mortality, adjusted for age and procedural indication, was significantly higher; however, reassuringly, a landmark analysis at 30 days showed that the mortality adjusted for age and procedural indication was no longer significant. However, hazard ratios for MI, re-PCI, ST, ISR and TLR were increased after the first 30 days (Table 1).
Due to improvements in covered stent features, a shift towards a newer stent graft was observed during the 12-year period. The GM was the only available covered stent at the start of the period, shifting to OU in the middle and ANU at the end of the period, and lastly to PAP.
Development of procedure complexity is shown in Supplementary Figure 1. There was an increase in procedures treating the left main, LAD and previous PCI patients.
Analysing differences between covered stent types showed that mortality during the procedure, at one month and one year was highest for the OU. Additionally, rehospitalisations for a new MI, re-PCI, ISR and TLR were all higher for the OU.
Of note, no ST was found for the equine pericardium covered stents OU and ANU during the entire follow-up period.
Discussion
Covered stents were rarely used – 0.13% (265/197,948) of stented PCI patients during the study period of 12 years. The short- and long-term outcomes for patients receiving a covered stent are significantly worse compared with all other PCI stented patients in terms of ISR, TLR, ST, death, MI, and re-PCI. This is unsurprising as covered stents are frequently used for sealing of iatrogenic and potentially fatal perforations with a poor outcome, as shown in contemporary registries1,7,20. Also, in SCAAR, a covered stent was used in fewer than one third of patients in case of a perforation with or without tamponade1,7,20. This low use of covered stents could be due to poor deliverability; they are bulky and stiff and only available in relatively large sizes (particularly at the beginning of the decade), which makes them risky and unsuitable to use in distal and small coronary arteries. Major side branches may also restrict their use, as a covered stent may occlude the side branch. Still, in the emergency of a perforation, a covered stent was deemed necessary and, accordingly, we found the incidence of side branch occlusion to be significantly higher. Occlusion of large side branches may increase infarct size and mortality.
We confirm the finding of others that women were more frequently treated with covered stents than the average PCI population. This may indicate that female coronary vessels are more prone to perforation, as they are frequently smaller and more tortuous1-3,20.
Besides the use of covered stents in perforations, covered stents may be used for exclusion of friable material and thrombus frequently found in saphenous vein grafts. This was an unclear indication at the beginning of our study period until the first randomised trial showed that it was futile10. Covered stents were occasionally used for other reasons than treating a stenosis, for example fistulas, septal ablation, pseudoaneurysm and aneurysms (Supplementary Table 1, Supplementary Table 2).
Other authors have reported an increase in the use of covered stents in a similar time period and argued that it may be due to a higher number of complex procedures1. We found that the percentage of procedures in the left main, LAD and previous PCI procedures increased slightly, whereas complex CTO procedures remained at a stable level (Supplementary Figure 1). However, as the overall number of procedures increased during the study period, the total number of complex procedures was higher (data not shown).
Comparisons among the four different covered stents must be interpreted with caution as the study was underpowered and unmeasured confounders may have affected outcomes. That said, the OU performed numerically worst in all examined parameters except for ST. The levels of ST for the GM and PAP were higher than for regular stents. Thrombogenicity and the potential lack of endothelialisation of a covered stent have been a concern since they entered the market; accordingly, it has been shown that the risk of ST is higher for covered stents than for other stents1,7,11,20. It is thus notable that both equine covered grafts (the OU and the ANU) were free from any incidence of ST in the same setting. This suggests the excellent properties of the equine pericardium with respect to ST compared to the synthetic materials used for the other covered stents. However, the otherwise negative outcome for the discontinued OU may be due to its need to be rinsed, which was time-consuming, and may have left a residue of glutaraldehyde, which may have added to the adverse events. When newer generations of covered stents (OU and ANU) were introduced, being less bulky they were expected to be more deliverable than the GM. It is therefore surprising that the procedural success was similar among these three types. Not until the PAP entered the market was an improved procedural success of >90% noted, probably because it is available in more sizes and has a lower profile and thus better deliverability, which again changed the covered stent landscape in Sweden.
This report from 2005-2017 from the national SWEDEHEART/SCAAR registry is the first attempt to elucidate the short- and very long-term outcome of patients treated with covered stents compared to the entire stented PCI population of a country. Patients receiving a covered stent have a worse outcome than other stented patients in terms of ISR, TLR, death, MI, and re-PCI. Periprocedural mortality is significantly higher, but a landmark analysis after one month, corrected for procedural indication and age, demonstrated long-term mortality thereafter to be equal to other stented patients. A notable observation was that equine pericardium covered stents did not have any stent thrombosis during the entire study period.
Limitations
Only covered stents that were implanted are included in the SCAAR. Totally unsuccessful attempts to implant a covered stent are not recorded as “a covered stent” procedure. This may partially explain the high procedural success rate.
Large differences between the covered stents were observed; however, because of possible concealed confounders which could not be adjusted for, this cannot be analysed. This, as well as insufficient power due to the small numbers in the groups, urges caution in interpreting differences as being no more than hypothesis-generating.
Registration of a complication in the registry refers to the whole entity of the procedure. It is therefore not certain whether a complication was due to the index procedure or due to the use of a covered stent. Duration of dual antiplatelet therapy (DAPT) is not recorded in the registry, which might have helped to elucidate the optimal duration of DAPT in patients treated with covered stents.
Another important factor affecting the results is the reporting hospital in which the procedure (and probably also the follow-up) was performed.
Conclusions
This observational study from the entire Swedish population shows that covered stents have a high incidence of procedural, one-month and long-term risk for adverse events as compared to all other stents. A landmark analysis after one month showed that the rate of long-term mortality, corrected for age and procedural indication, is similar compared to other PCI stent-treated patients up to 12 years after the index procedure.
| Impact on daily practice Patients receiving covered stents have a high rate of adverse events and mortality both in the short as well as in the long term, compared to all other stented patients. However, after the initial phase, mortality appears to be similar to other stented patients. In patients with a high risk of stent thrombosis, the equine pericardium covered stent is superior to other covered stents. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. Development in procedure complexity 2005-2016.
Supplementary Table 1. Background and procedural characteristics.
Supplementary Table 2. Background characteristics of lesion type and segment.
To read the full content of this article, please download the PDF.

