
Abstract
Aims: Transcatheter interventions with balloon-expandable valves have been shown to be efficacious for the treatment of mitral annuloplasty failure but are limited by the fact that there is no opportunity for post-implantation adjustment. The aim of this study was to assess the safety and efficacy of the fully repositionable and retrievable Direct Flow Medical (DFM) valve for the treatment of mitral annuloplasty failure.
Methods and results: Patients who underwent transcatheter mitral valve-in-ring (VIR) implantation of a DFM valve for failed mitral annuloplasty deemed high risk for redo surgery were included at four institutions. Eight patients underwent transcatheter mitral VIR procedures with implantation of the DFM valve. The DFM prosthesis was successfully positioned in all patients. Two patients required retrieval of the device due to a suboptimal result, and a further patient required repositioning of the valve with an ultimately successful implantation. During the 30-day follow-up period, two patients died for reasons unrelated to the valve implantation. The four patients with successful implantation had normal valve function associated with a significant improvement in their functional status.
Conclusions: For the first time, we demonstrate the safety, efficacy and advantages of using the DFM prosthesis for the treatment of mitral annuloplasty failure.
Abbreviations
DFM: Direct Flow Medical
LV: left ventricle
LVOT: left ventricular outflow tract
MR: mitral regurgitation
MSCT: multi-slice computed tomography
PVL: paravalvular leak
TEE: transoesophageal echocardiography
VIR: valve-in-ring
Introduction
Despite advances in surgical techniques for mitral repair, approximately 25% of patients require repeat intervention within 10 years for severe mitral regurgitation due to ring annuloplasty failure1. Whilst redo surgery is the treatment of choice, this approach is associated with significant morbidity and mortality, especially in this patient group that often has multiple associated comorbidities2. As a consequence, the use of less invasive techniques is particularly attractive.
Transcatheter interventions have been previously described for the treatment of mitral annuloplasty failure and have been shown to be efficacious when performed in carefully selected patients with acceptable short-term outcomes3-6. However, a number of technical factors need to be taken into consideration when contemplating this therapeutic approach, including optimal valve positioning, valve stability, the risk of left ventricular outflow tract obstruction and the occurrence of paravalvular leak (PVL). The experience to date for mitral transcatheter valve-in-ring (VIR) treatment has been only with the use of balloon-expandable (BE) devices, which are limited by the fact that the operator has one single attempt at correct positioning with no opportunity for post-implantation adjustment. Therefore, the possibility of using a device that is repositionable and fully retrievable is appealing to reduce complications and improve the likelihood of procedural success.
The aim of this study was to report our preliminary multicentre first-in-man experience with transcatheter mitral VIR using the fully repositionable and retrievable Direct Flow Medical® (DFM) device (Direct Flow Medical Inc., Santa Rosa, CA, USA) for the management of mitral annuloplasty failure.
Methods
PATIENTS
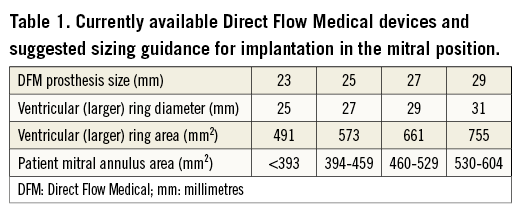
The population consisted of patients who underwent transapical transcatheter mitral VIR implantation of a DFM for failed mitral annuloplasty at four institutions (San Raffaele Scientific Institute, Milan, Italy; Albertinen Heart Center, Hamburg, Germany; SHG Kliniken Völklingen, Völklingen, Germany; Niguarda Hospital, Milan, Italy). All patients underwent transoesophageal echocardiography (TEE) and multi-slice computed tomography (MSCT) evaluation to determine suitability and prosthesis sizing. Currently available DFM prosthesis sizes and their suggested sizing recommendations for implantation in the mitral position are summarised in Table 1. To ensure the greatest likelihood of valve stability and optimal sealing we aimed for at least 25% oversizing on the basis of the ratio of the area of the larger ring that results in sealing on the ventricular aspect of the mitral annulus (e.g., 27 mm ring for a 25 mm device) and the mitral ring area. The decision to perform the intervention was made following discussion by the “Heart Team” in patients considered to be high risk for redo surgery. Informed consent for the procedure was obtained from all patients, who were also informed about the potential risks and benefits related to the off-label use of the DFM device for this indication.
PROCEDURE
Procedures were carried out under general anaesthesia with the aid of TEE guidance. All implantations were performed via the transapical access route using established surgical techniques. After crossing the mitral valve and placing a stiff wire in the right inferior pulmonary vein or left atrium (LA), a 22 or 24 Fr sheath was inserted into the left ventricle (LV). The aim for positioning was to cover enough of the ring with the 2 mm larger ventricular ring to avoid PVL but for the device not to be too ventricular, which could result in LVOT obstruction.
The DFM transcatheter valve system was inserted into the LA, unsheathed with subsequent inflation of both rings in the LA. The ventricular ring was then deflated and the prosthesis pulled towards the LV until the ventricular ring was within the LV and the inflated atrial ring was parallel to the mitral annulus. The ventricular ring was then inflated and, once the valve was thought to be in an optimal position, TEE was performed to assess the presence or absence of central or paravalvular regurgitation and also to exclude LVOT obstruction. If the valve position was thought to be suboptimal, it was repositioned to a more acceptable position or completely retrieved if an acceptable position was not achievable. If the position was acceptable, the valve was then definitively deployed by polymer exchange and detached as previously described7. The procedure is illustrated in Figure 1.
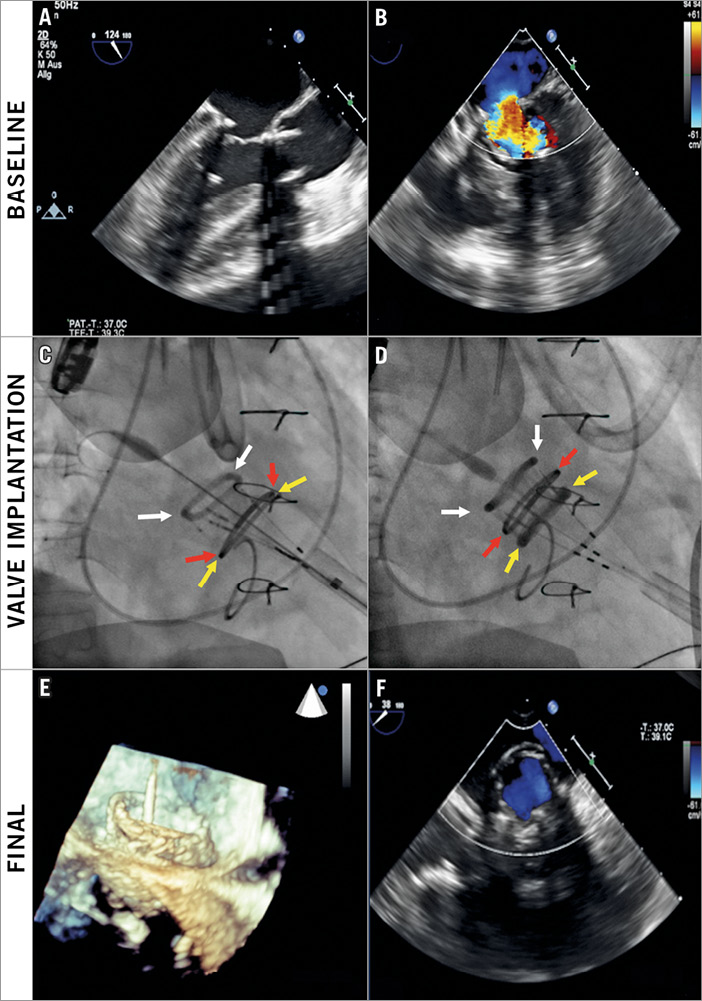
Figure 1. Representative valve-in-ring implantation. Baseline transoesophageal echocardiogram (TEE) demonstrating severe mitral regurgitation (MR) secondary to mitral annuloplasty failure (A, B). Implantation of valve (C, D) with positioning of the valve with the aid of fluoroscopy (C) with one ring inflated in the atrium (white arrows), the mitral ring (red arrows) and the second ring inflated in the ventricle (yellow arrows). Final TEE appearance of valve-in-ring (E) with no residual MR (F).
FOLLOW-UP
Clinical follow-up and echocardiography were performed prior to patient discharge. All adverse events were recorded prospectively. Subsequent patient follow-up was carried out by the host institution or by the patient’s own cardiologist with the data collected by telephone calls.
STATISTICS
Data are expressed as mean±standard deviation (SD). Statistical analysis was performed using SPSS, Version 21.0 (IBM Corp. Armonk, NY, USA).
Results
PATIENTS
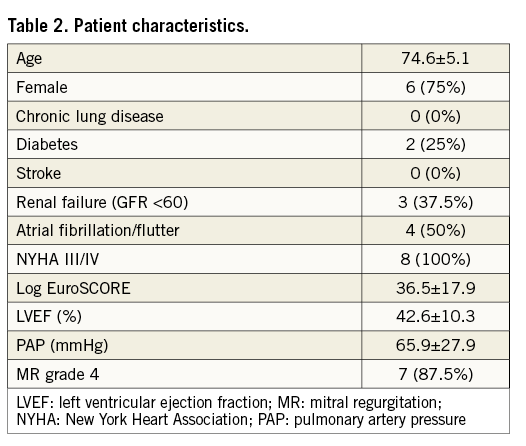
Between April 2014 and April 2015, in four European centres, eight patients underwent transcatheter mitral VIR procedures with implantation of the DFM valve. Patient characteristics are illustrated in Table 2.
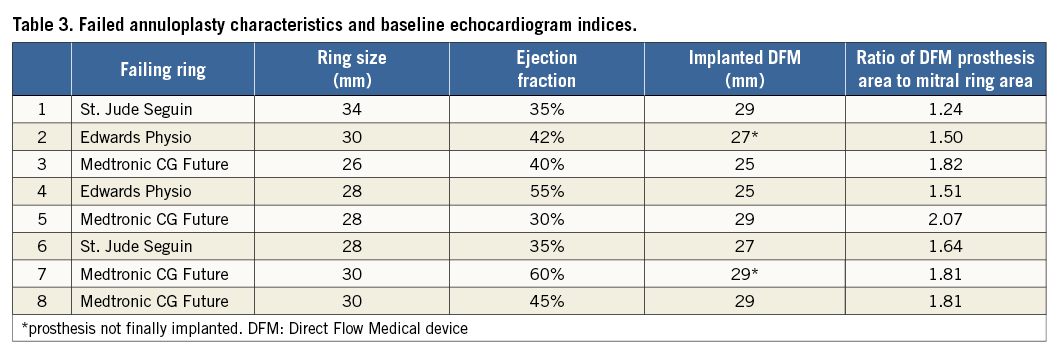
Annuloplasty rings were semi-rigid in all patients (Table 3). The manufacturer’s diameters ranged from 26 mm to 34 mm. Baseline echocardiography confirmed severe regurgitation in all eight cases. The DFM device was selected on the basis of pre-procedural CT imaging and size of the in situ mitral valve ring with the TAVI prosthesis oversized by 1.67±0.26 times in relation to the mitral ring (Table 3).
PROCEDURE AND IMMEDIATE OUTCOME
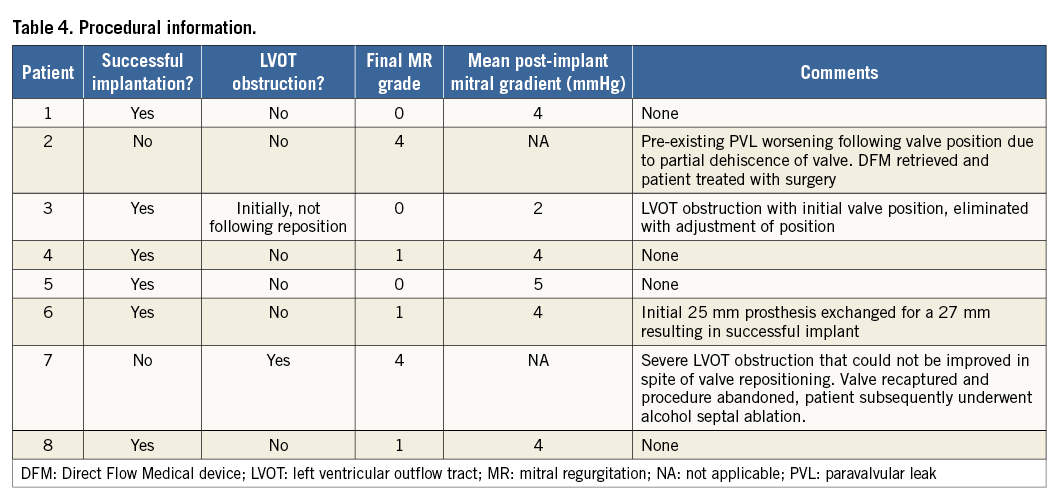
Procedural specifics with regard to each patient are summarised in Table 4. The DFM prosthesis was successfully positioned in all eight patients. In one patient, pre-existing mild PVL deteriorated following positioning of the prosthesis confirming the pre-op suspicion of partial dehiscence of the ring. The DFM device was recaptured and the patient was treated electively with conventional surgery without complication. Two patients were noted to have severe LVOT obstruction following initial positioning of the device. In one patient, the LVOT obstruction was eliminated with repositioning of the valve, resulting in a successful implantation. However, this was not possible in the other patient. The device was recaptured and the patient was managed conservatively. One patient required exchange of a 25 mm device to a 27 mm prosthesis to decrease residual PVL, and the remaining three patients underwent successful implantation. Of the six patients in whom a DFM device was successfully implanted, no patient demonstrated a significant transmitral valve gradient (3.8±0.98 mmHg) (Table 4).
FOLLOW-UP
During the follow-up period, two patients died. The first suffered an asystolic cardiac arrest eighteen days following the procedure, suffering hypoxic cerebral damage. The second had severe left ventricular dysfunction before the procedure and developed acute renal failure on the third day following the procedure. She refused dialysis and died from heart failure.
At 30-day follow-up, the four patients with successful implantation of a DFM in the mitral position remained well, with normal valve function as assessed by transthoracic echocardiography associated with a significant improvement in their functional status (NYHA I-II).
Discussion
This first-in-man study of a VIR procedure using the repositionable and retrievable DFM device in selected patients who are prohibitively high risk for redo surgery demonstrated feasibility and safety.
The experience with VIR procedures to date has been exclusively with the use of BE devices3-5. BE valves are limited by the fact that this is a “one shot procedure” with no opportunity to adjust or recapture the device in the setting of suboptimal results. The two key advantages of the DFM device are the ability to reposition the device and the ability to recapture it completely. This may be particularly helpful in the setting of radiolucent rings where fluoroscopy is unhelpful in guiding valve positioning.
Indeed, in our case series, these features enabled us in one patient to reposition the device prior to final deployment and in another patient to exchange the device for a larger size, resulting in an ultimately successful implantation. In two further patients, in the setting of a ring dehiscence in one case and of haemodynamic instability because of severe LVOT obstruction in the other case, valves were completely recaptured, thereby preventing a catastrophic outcome for both patients.
As opposed to the treatment of aortic bioprosthesis failure, where the sewing ring of the prosthesis is circular, mitral annuloplasty rings are usually asymmetrical (D-shaped). By virtue of its non-metallic design, the DFM device is able to conform to the non-circular shape of the mitral ring and therefore to increase the likelihood of less residual regurgitation following implantation in comparison to BE valves. Inevitably, the valve will be more asymmetrical in comparison to BE valves, although the impact of this with regard to long-term durability remains uncertain.
Limitations
There are some limitations to this case series. The numbers of patients were small and the patients were highly selected. Furthermore, follow-up duration was limited. Finally, the experience described here has been exclusively in the management of patients with semi-rigid mitral rings; these results may not be readily applicable for the management of mitral annuloplasty failure in the presence of rigid rings.
Conclusion
We, for the first time, demonstrate the safety, efficacy and advantages of using the fully repositionable and retrievable Direct Flow Medical prosthesis for the transcatheter treatment of mitral annuloplasty failure in patients who are deemed to be at prohibitively high risk for redo surgery.
| Impact on daily practice Increasing numbers of patients are presenting with symptoms associated with mitral annuloplasty failure. Redo surgery is the treatment of choice; however, this is associated with significant risk. Our experience demonstrates that valve-in-ring treatment with the Direct Flow Medical device is safe and efficacious, with the added advantage of being repositionable to optimise final valve position prior to definitive deployment or completely retrievable in the setting of the inability to obtain an acceptable procedural result. |
Conflict of interest statement
A. Latib serves on a Medtronic advisory board and is a consultant for Direct Flow Medical. A. Colombo is a minor shareholder in Direct Flow Medical. F. De Marco is a consultant for Direct Flow Medical. G. Bruschi is a proctor for Medtronic. F. Gatto is a proctor for Direct Flow Medical. The other authors have no conflicts of interest to declare.

