Abstract
Aims: To study the feasibility and safety of the non-metallic, repositionable and retrievable percutaneous Direct Flow Medical (DFM) aortic valve.
Methods and results: The first-generation (22 Fr) DFM valve has been evaluated in a prospective non-randomised trial in 31 high-risk patients with severe symptomatic aortic stenosis. The procedural success rate was 71%, 30-day mortality 12.9%. Survival at three years was 60% and all patients had none/trace aortic regurgitation at three years. Based on the initial experience, an 18 Fr device has been developed with several important revisions to improve the efficacy and safety of the procedure. Currently, it is being evaluated in a multicentre non-randomised trial which will include 100 patients. The primary endpoint is freedom from all-cause mortality at 30 days.
Conclusions: The 22 Fr DFM valve has been successfully assessed in a first-in-man feasibility and safety trial. Up to three-year follow-up sustained clinical benefit and haemodynamic performance was demonstrated with no or trace aortic regurgitation in all patients. The 18 Fr DFM valve is under investigation in an on-going trial.
Description of the valve
The Direct Flow Medical (DFM) aortic valve (Direct Flow Medical, Santa Rosa, CA, USA) (Figure 1) is a non-metallic percutaneous valve with an inflatable ring cuff frame designed to encircle and capture the native valve annulus, thereby ensuring anchoring of the bioprosthesis and minimising potential paravalvular leaks, dislodgement or migration.
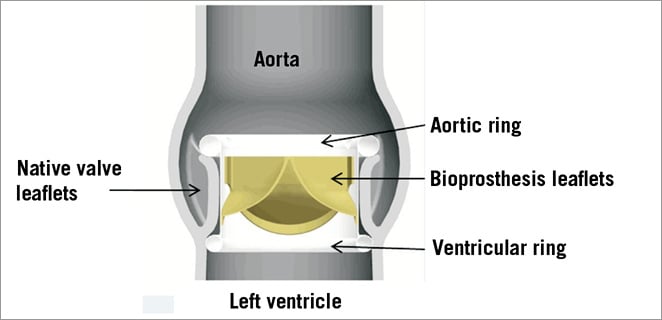
Figure 1. Direct Flow Medical aortic valve in native aortic annulus with individually inflatable aortic and ventricular ring.
The tricuspid bovine pericardial valve is attached to a polyester fabric cuff which conforms to the native aortic annulus. An upper (aortic) and lower (ventricular) ring balloon interconnected by a tubular bridging system can be inflated independently through two of the three position-fill lumens.
The 18 Fr DFM bioprosthesis is available in 25 mm and 27 mm sizes. It is designed to be fully repositionable and retrievable prior to final deployment through the introducer.
Delivery system
The delivery system is configured with multi-axial tubes, including one retractable outer sheath, one multi-lumen assembly, one inner lumen assembly and three position wires (Figure 2). The distal end of the three position wires of the delivery system are connected to the bioprosthesis via a nylon thread located at the proximal end. All three position wires are used to position and align the inflatable ring frame in the native annulus.
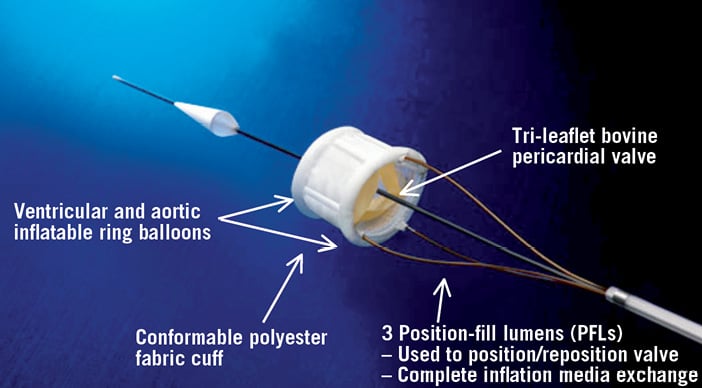
Figure 2. The Direct Flow Medical valve delivery system. The 18 Fr valve delivery system contains three position-fill lumens which are attached to the bioprosthesis. Two of these position-fill lumens are used to inflate and deflate the ring balloons and all three are used to position the bioprosthesis.
Implantation procedure
Following balloon valvuloplasty, the DFM delivery system is advanced until the implant housing is positioned in the left ventricle. The outer sheath is retracted and both ring balloons are inflated by injecting a mixture of saline and contrast agent through the position wires. Immediately after balloon inflation the valve is functioning, there is no interruption of blood flow and no need for rapid pacing.
The aortic ring balloon is then deflated and the prosthesis is positioned by individually pulling and/or pushing the three position-fill lumens attached to the prosthesis to align the lower (ventricular) ring balloon to the aortic annulus.
When the desired position is achieved, the aortic ring balloon is inflated to fix the prosthesis. The haemodynamic performance (paravalvular leak and transvalvular gradient) and correct position are then assessed by echocardiography and aortography. If the valve is not in the optimal location, the balloons can be deflated and the prosthesis then repositioned or even completely retrieved (see below).
When an optimal result is achieved, the saline-contrast mixture is replaced by a polymer which becomes solid within 90 minutes and maintains the implant permanently in position. The procedure is completed by detachment of the position-fill lumens (Figure 3).
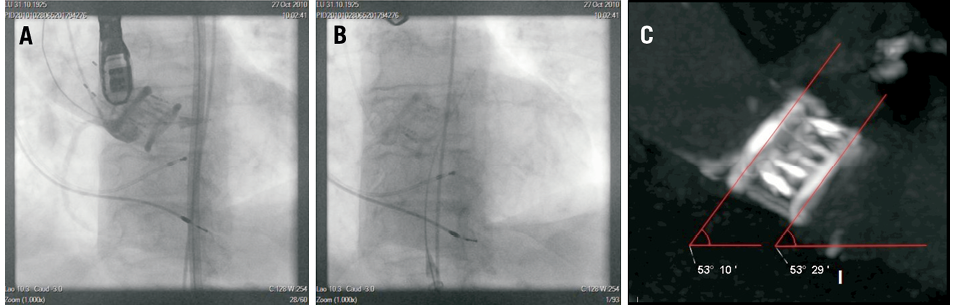
Figure 3. 18 Fr DFM valve implantation, two-month CT follow-up. Aortography with the DFM valve in final position with the three position-fill lumens attached to the prosthesis (A). Implanted DFM valve after polymer exchange and detachment of the position-fill lumens (B). Dual-source multislice CT two months after DFM valve implantation (C)
Retrieval procedure
If retrieval of the prosthesis is necessary, both aortic and ventricular ring balloons are deflated and the valve is pulled back to the tip of the introducer sheath. The retrieval system (Figure 4) is advanced and a nitinol basket is deployed in the abdominal aorta. The prosthesis is pulled into the basket which is then pulled into the introducer sheath.
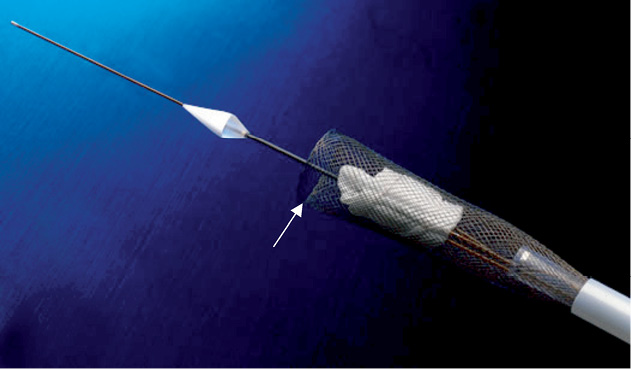
Figure 4. Retrieval system with the nitinol mesh basket for the 18 Fr DFM valve. For retrieval a nitinol mesh basket is deployed in the abdominal aorta. The deflated prosthesis is pulled into the basket which is then pulled into the introducer sheath.
Current status
The first-generation DFM aortic valve, a 22 Fr system, has been evaluated in a prospective, non-randomised clinical trial at two centres in Germany conducted between October 2007 and August 2008.
The purpose was to determine the feasibility and safety of the device in patients with severe symptomatic aortic valve stenosis at high surgical risk (logistic EuroSCORE ≥20%). Thirty-one patients were enrolled in the study1,2, 15 male and 16 female, mean age 84±4 years. Procedural success rate was 71%. The main reason for a failed implantation was excessive calcification of the native aortic valve. Thirty-day all-cause mortality was 12.9% in this first-in-man trial. The majority of patients had no paravalvular aortic leak.
For the 18 patients who were discharged with permanent implants, three-year follow-up data are available. Survival at three years was 60% which is well within the range of what has been published for commercially available percutaneous aortic valves (Figure 5). At three years, 50% (n=5) of patients were in NYHA Class I, the remaining 50% (n=5) were in NYHA Class II, transvalvular gradient was 25.9±12.3 mmHg (mean±SD). Of particular note, at three years all patients (n=10) had none/trace aortic regurgitation.
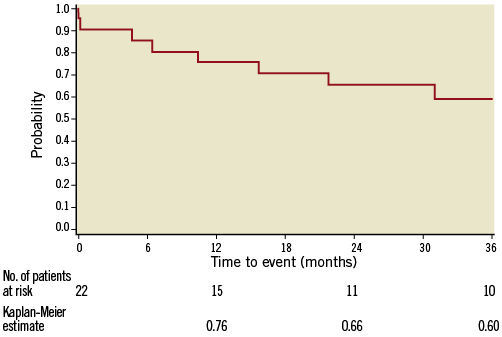
Figure 5. Kaplan-Meier survival curve. Kaplan-Meier survival curve of patients treated with the 22 Fr DFM aortic valve prosthesis.
Because of the non-metallic design of the DFM valve, there was concern about valve performance and recoil over time. Therefore, in a subset of patients, the long-term performance of the DFM aortic valve was evaluated by sequential dual-source multislice CT and echocardiographic assessment over two years, revealing stability of the position, shape and haemodynamic performance3.
The new 18 Fr DFM valve
Based on the initial experience with the first-generation device, several important alterations have been made in order to increase the efficacy and safety of the procedure and to simplify the intervention.
Apart from the reduction in profile to 18 Fr, those features are: increased radial force, improved positioning by stiffer position-fill lumens allowing advancement and retraction, simplified valve retrieval, improved balloon performance, improved polymer exchange system with pressure and volume feedback, and simplified valve loading.
Since January 2012, the second-generation 18 Fr DFM valve system has been under clinical investigation in a multicentre non-randomised trial, the DISCOVER trial, with planned enrolment of 100 patients. Patients have to have a log EuroSCORE ≥20 or other high surgical risk features not reflected by the log EuroSCORE. The primary endpoint is freedom from all-cause mortality at 30 days. The secondary safety and efficacy endpoints are according to the VARC criteria. All data are evaluated by an independent core laboratory for echocardiography as well as for angiography and have clinical event committee adjudication.
Advantages and shortcomings of the DFM aortic valve
The DFM valve incorporates several advantages compared to CE mark percutaneous aortic valve prostheses. Due to the non-metallic valve design, the delivery system is very flexible. There is no interruption of flow and no need for rapid pacing during positioning and deployment of the valve. The inflatable polyester cuff conforms to the native aortic annulus which minimises paravalvular leaks. The three position-fill lumens allow precise positioning by pulling and/or pushing. The valve can be repositioned and retrieved even after final valve deployment. Repositionability allows for assessment and optimisation of the haemodynamic outcomes prior to final device deployment. Complications such as valve embolisation can be avoided and other complications such as device mismatch and coronary occlusion can be managed, which increases the safety of the TAVI procedure.
Extensive calcification of the LVOT can make positioning of the DFM valve difficult.
Conflict of interest statement
J. Schofer, R. I. Low and E. Grube received consultancy fees from Direct Flow Medical, Inc., Santa Rosa, CA, USA. K. Bijuklic and T. Tübler have no conflicts of interest to declare.

