
Abstract
Aims: Our aim was to assess the performance of the Direct Flow Medical (DFM) repositionable and retrievable transcatheter aortic valve implantation (TAVI) system in high-risk patients with severe aortic stenosis.
Methods and results: One hundred and five consecutive high-risk patients with severe aortic stenosis undergoing TAVI with the DFM prosthesis were enrolled in six high-volume TAVI centres in Germany, Italy, The Netherlands, Belgium, and Luxembourg. The “inner curve” technique was systematically used in all patients. The primary endpoint was all-cause mortality at 30 days of clinical follow-up. Secondary endpoints for the same time frame were: (i) VARC-2-defined patient safety; and (ii) VARC-2-defined device success. The primary endpoint of all-cause mortality at 30 days was met in 1.9% (two patients). The VARC-2-defined device success rate was 98.1%. The combined patient safety endpoint was met in 88.6%. Residual moderate aortic regurgitation was observed in 1.9% (two patients). Permanent pacemaker implantation due to post-procedural persistent advanced atrioventricular block was performed in 9.5% (10 patients).
Conclusions: In a multicentre, real-world clinical setting of high-risk patients with severe aortic stenosis, a repositionable and retrievable TAVI system was effective and safe in the short-term follow-up.
Introduction
Transcatheter aortic valve implantation (TAVI) is well established as a percutaneous treatment option in patients with severe aortic stenosis, who are at prohibitive or high surgical risk1-10. Optimal valve positioning during the procedure is key to avoiding significant residual paravalvular aortic regurgitation (AR), which is a major predictor of worse clinical outcome11-13. Additionally, the inability of early-generation TAVI systems to be retrieved and repositioned led to severe procedural complications, such as occlusion of coronary ostia or prosthesis embolisation. These adverse events often required bail-out percutaneous or surgical interventions14-17.
The introductory DISCOVER trial showed high rates of patient safety and procedural efficacy in patients with severe aortic stenosis treated with the repositionable/retrievable Direct Flow Medical® (DFM) prosthesis (Direct Flow Medical, Inc., Santa Rosa, CA, USA)18. The DFM TAVI system, with its non-metallic, inflatable and deflatable structure, allows precise positioning, retrieval and assessment of valve performance in its final position. More recently, the deployment of the prosthesis has been standardised, with the use of the “inner curve technique”19. Therefore, in the present study we sought to assess the efficacy and safety profile of the DFM prosthesis in a real-world, high-risk patient population with severe aortic stenosis, in which a similar implantation technique was systematically applied.
Methods
PATIENT POPULATION AND DEFINITIONS
This is a multicentre, observational study, which enrolled consecutive patients with severe aortic stenosis with high or prohibitive surgical risk undergoing TAVI with the DFM prosthesis from March to November 2013. The primary endpoint of the study was all-cause mortality at 30 days. Secondary endpoints were: (i) VARC-2-defined patient safety at 30-day follow-up (a composite including freedom from death, myocardial infarction, stroke, stage 3 acute kidney injury, and major vascular complications); and (ii) VARC-2-defined device success (composite of successful vascular access, delivery and deployment of the device and successful retrieval of the delivery system, correct position of the device in the proper anatomical location, intended performance of the prosthetic heart valve [mean aortic valve gradient <20 mmHg or peak velocity <3 m/s, without moderate or severe prosthetic valve AR], and single prosthesis implantation). Post-procedural AR and clinical events were not centrally collected, but were site-reported.
All potential TAVI candidates were assessed by a local Heart Team composed of interventional cardiologists and cardiac surgeons who determined the indication of the patient for TAVI. All candidates underwent a pre-interventional screening process to determine eligibility. Coronary anatomy was evaluated by coronary angiography. Valvular anatomy was assessed using transthoracic and transoesophageal echocardiography and multi-slice computed tomography (MSCT) of the thoracic aorta. Vascular access was evaluated using MSCT of the abdominal aorta, iliac and femoral arteries. Severe aortic valve stenosis was defined by echocardiographic criteria including a mean gradient >40 mmHg or peak jet velocity >4.0 m/s and aortic valve area ≤0.8 cm2 or aortic valve area index ≤0.5 cm2/m2.
Baseline surgical risk was estimated with the logistic EuroSCORE. Exclusion criteria were: an annulus diameter derived from perimeter by multi-detector computed tomography (MDCT) <19 mm or >26 mm; prior valve surgery; a prosthetic heart valve in any position; myocardial infarction or coronary intervention within 30 days prior to the index procedure; stroke or transient ischaemic attack within six months. As opposed to the CE-mark DISCOVER trial18, patients with left ventricular ejection fraction (LVEF) <30%, significant mitral insufficiency, or chronic kidney disease were not excluded.
DEVICE AND PROCEDURE DESCRIPTION
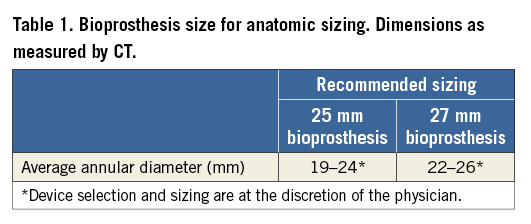
The DFM prosthesis is a non-metallic percutaneous bovine pericardial valve with an expandable Dacron polyester double ring design containing non-compliant angioplasty balloon technology20. The upper (aortic) and lower (ventricular) ring, interconnected by a tubular bridging system, can be pressurised independently through position-fill lumens (Figure 1). At the time of this study, valve sizes available included a 25 mm size for annular diameters of 19-24 mm and a 27 mm size for 22-26 mm (Table 1).
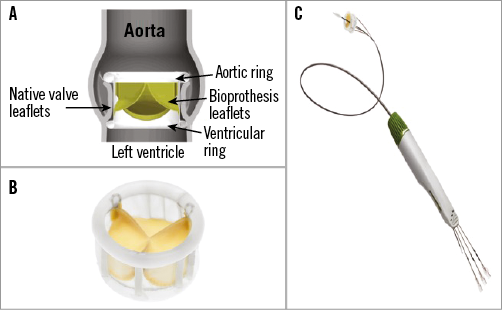
Figure 1. Direct Flow Medical bioprosthesis and delivery system. A) The Direct Flow Medical valve is a bovine pericardial valve with an expandable Dacron polyester double ring design. The upper (aortic) and lower (ventricular) non-compliant ring balloons are interconnected by a tubular bridging system. B) Direct Flow Medical bioprosthesis. C) Transfemoral delivery system.
After balloon aortic valvuloplasty (BAV), the DFM delivery catheter is positioned in the left ventricle. The two rings are pressurised by injecting a mixture of saline and contrast media through the position-fill lumens up to 12 atm. After deflation of the aortic ring, the operator retracts and/or advances the three position wires, ensuring ventricular ring alignment to the aortic annulus, followed by aortic ring balloon pressurisation. The most commonly used technique is the “inner curve technique”19 which aligns the ventricular ring at the inner curve of the aortic arch first by pulling on the wire closest to this position (Figure 2). Once this position is achieved, pulling on the other two wires “closes the door” and aligns the entire ventricular ring with the aortic annulus. After assessment of the valve haemodynamics, the valve can be permanently implanted or can be depressurised for prosthesis repositioning or completely retrieved. In the latter case, both rings are deflated and the valve is pulled into a nitinol basket in the abdominal aorta and retrieved through the introducer sheath. When an optimal position has been obtained, a polymer is infused into the bioprosthesis replacing the contrast and saline while maintaining the pressure in the bioprosthesis at 12 atm. The polymer solidifies and the device is permanently implanted18,20.
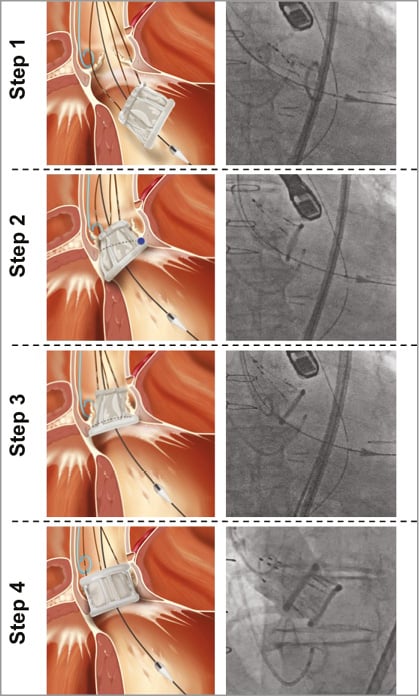
Figure 2. Case example illustrating valve positioning and “inner curve” technique. Step 1 (valve orientation). The DFM bioprosthesis is unsheathed and unfolded in the LV cavity. Step 2 (valve tracking). The positioning wire corresponding to the inner curvature of the ascending aorta is pulled up to the aortic annulus. Step 3 (“Closing the door” phase). Outer curve side of valve pulled up to the aortic annulus, maintaining tension to the inner curvature positioning wire (used as hinge). Step 4 (valve inflation and final positioning). The upper ring of the DFM bioprosthesis is inflated. DFM: Direct Flow Medical; LV: left ventricle
STATISTICAL ANALYSIS
Categorical data are presented as frequency (percentages). Continuous variables are expressed as mean±SD. Results for effective orifice area, mean gradient, left ventricular ejection fraction, and AR were reported from post-procedure up to 30 days by taking the first available data point. Data were analysed with SPSS, Version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
PATIENT POPULATION
Baseline demographic and clinical characteristics of the global population are shown in Table 2. The majority of the enrolled patients had advanced NYHA symptoms (NYHA III-IV in 75 patients [71%]) and 19 (18%) had a severely reduced left ventricular function (LVEF <35%). Chronic kidney disease (defined as pre-procedural glomerular filtration rate <60 ml/min/1.73 m2) was present at baseline in 34 patients (32%).
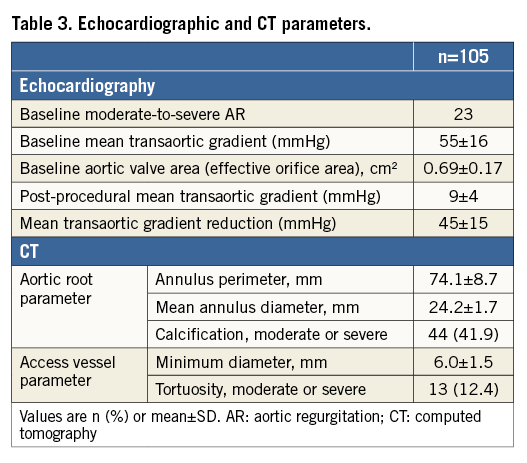
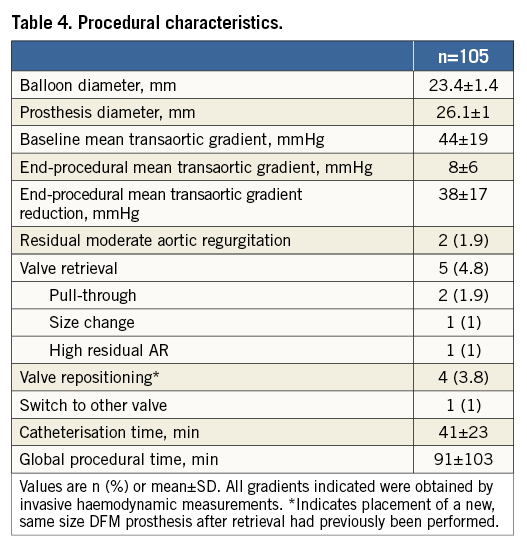
Echocardiographic/CT measurements and procedural characteristics are reported in Table 3 and Table 4, respectively. A significant reduction in transvalvular aortic gradient was observed both in echocardiographic (55±16 vs. 9±4 mmHg; p<0.001; mean gradient reduction=45±15 mmHg) and intraprocedural invasive haemodynamic evaluation (44±19 vs. 8±6 mmHg; p<0.001; mean gradient reduction=38±17 mmHg). In one patient, a 29 mm CoreValve® (Medtronic, Minneapolis, MN, USA) was implanted due to residual AR after attempted DFM implantation. The CoreValve implantation required multiple post-dilatations with moderate final paravalvular AR. Residual moderate AR was observed in only two patients (1.9%), including the CoreValve implant.
STUDY ENDPOINTS
The primary endpoint of the study (all-cause mortality at 30 days) was observed in two patients (1.9%) (Table 5).
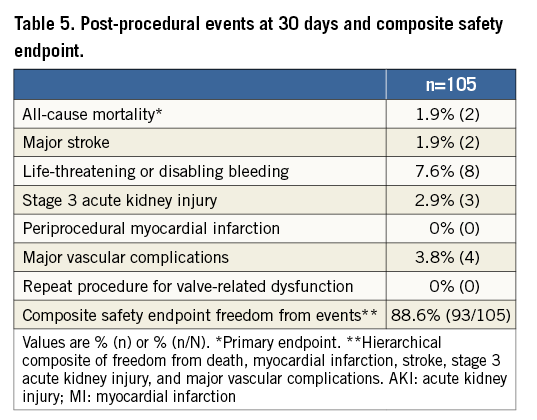
The patient safety endpoint freedom from events was successfully met in 94 out of 105 patients (89.5%) (Table 5). VARC-2-defined major vascular complications which required the intervention of the vascular surgeon were observed in four cases. The rate of pacemaker implantation for all 100 patients was 9.5%. In one case (1.0%), cardiac tamponade was observed: the patient was treated with pericardial puncture and drainage, discharged after nine days from hospital admission, and remained uneventful up to 30 days of follow-up.
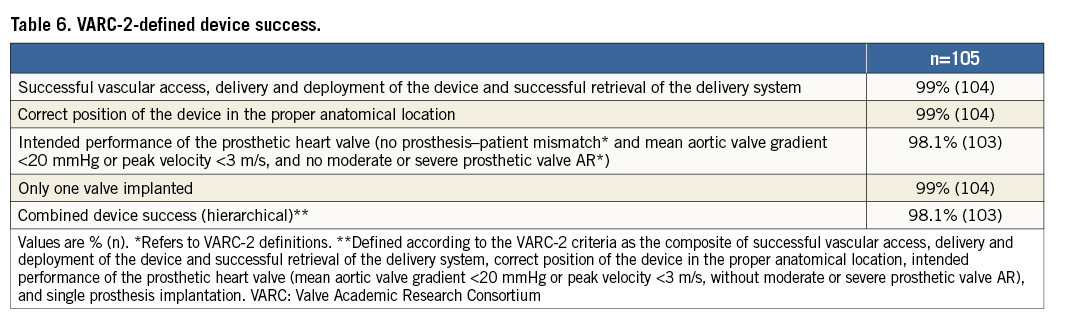
Device implantation was adjudicated as successful in 103 cases (98.1%) (Table 6). Device failure was due to a post-implant transvalvular gradient ≥20 mmHg or peak velocity ≥3 m/s in two patients. No patient-prosthesis mismatch was observed. Valve retrieval was performed in five cases (4.8%). The reasons for performing a valve retrieval were: pull-through in the aortic annulus during positioning (three cases related to positioning difficulties, with a successful implant with the second valve; 1.9%); size change (one case, 1%); and unacceptable residual AR (one case, 1%). In the first four cases, valve repositioning was performed, while, in the other case, the operator deemed it necessary to switch to another transcatheter aortic prosthetic valve (CoreValve 29 mm) due to persistent AR despite multiple positioning manoeuvres.
Discussion
The main findings were as follows: (i) in a real-world, high-risk patient population of patients with severe aortic stenosis treated with the DFM repositionable and retrievable TAVI system, freedom from all-cause mortality at 30 days was high at 98.1%; (ii) the rate of residual AR was notably low as assessed at the end of the procedure; and (iii) the device efficacy, as assessed by the device success and early safety rates, was high.
Recently, the FRANCE 2 prospective registry reported 30-day mortality rates as high as 8.5% for the transfemoral access patient cohort21. The mortality rate was lower in the SOURCE registry (6.3% for the transfemoral vascular approach) which utilised only the SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA)7. Conversely, the PARTNER randomised trial, enrolling patients using both the transfemoral and transapical approach, reported 30-day all-cause mortality rates of 3.4% for high-risk patients and 5% for inoperable patients with severe aortic valve stenosis22,23. Furthermore, a recent meta-analysis by Genereux et al reported a 30-day mortality rate of 7.8% for a series where both alternative vascular accesses and transfemoral access were used24. Second-generation TAVI systems such as the Direct Flow device report lower mortality rates. This has also been noted for the Edwards SAPIEN 3 (Edwards Lifesciences) and the Lotus valve (Boston Scientific, Marlborough, MA, USA), reporting 2.1% and 4.2%, respectively. This reflects the evolution in newly developed TAVI systems.
The current study observed higher device success and safety rates, as compared to previous experience with earlier-generation transcatheter prostheses. The high device success rate and safety profile is probably responsible for the low rate of 30-day all-cause mortality observed in the present study. In particular, we noted a relatively low degree of major vascular complications compared to first-generation transcatheter valve replacement systems. In our study, the observed major vascular complication rate was 3.8%, as compared to transfemoral rates that ranged from 5.5% in the FRANCE 2 registry to up to 15.3% in the PARTNER trial25. Since the vascular access used by the DFM system is 18 Fr (similar to CoreValve), the low rate of vascular complications in the present cohort may also be related to an improved technique with vascular access management rather than to the study device itself.
Additionally, there was no case of device embolisation. This can be attributed to the retrievability and repositionability features of the DFM prosthesis. Of note, recent findings from the U.S. PARTNER trial suggest that device embolisation is associated with an excess in mortality14.
The observed rate of moderate residual AR was very low in this patient cohort at 1.9%. In comparison, the recent Genereux et al meta-analysis reported a residual moderate-to-severe AR rate of 7.4%24. The post-TAVI new pacemaker implantation rate was 10%, as compared to 24-29% with the CoreValve and 5-11% with SAPIEN21,24,26. As compared to the DISCOVER trial, this registry observed a lower number of new pacemaker (PM) implantations18. We can hypothesise that this finding may be due to the following: (i) a higher prevalence of previously implanted PM in our study population; (ii) standardised, improved implantation technique (“inner curve”)19 for the entirety of the present study’s patient population; and (iii) increased experience with the DFM TAVI system in some of the enrolling centres that participated in the initial DISCOVER trial.
We observed only one prosthesis retrieval due to a sizing error. This finding demonstrates a clear and reliable understanding of prosthesis sizing issues, and can be interpreted as an indicator of the acquired experience of the participating centres with optimal DFM prosthesis sizing before the TAVI index procedure.
Study limitations
We acknowledge several limitations in the current study. As an observational, retrospective analysis, the findings might be subject to selection bias. In addition, we present only short-term (30-day) follow-up data. Accordingly, we cannot exclude that these findings may differ in the long-term follow-up.
Furthermore, risk stratification was performed using the logistic EuroSCORE at baseline, following the current clinical practice of the enrolling centres. This risk model has however previously been associated with overestimation of mortality risk, and currently the use of different risk stratification tools such as STS or EuroSCORE II is recommended27,28.
As an investigator-driven observational study, event and aortic regurgitation adjudication was site-reported. Accordingly, no core lab analysis has been performed. Although transoesophageal echocardiography and MSCT were systematically performed on-site as part of the sizing process, these data were not systematically analysed. We also acknowledge the absence of a comparator group to demonstrate efficacy compared to other devices.
Conclusions
In this real-world experience, TAVI, performed in a high-risk patient cohort with severe aortic stenosis using a repositionable/retrievable valve prosthesis, demonstrated low 30-day all-cause mortality and was associated with high rates of device success and efficacy. We found a very low incidence of residual paravalvular aortic regurgitation. These initial findings require larger patient cohorts and extended follow-up to draw more definitive conclusions.
| Impact on daily practice We present our real-world, multicentre, observational experience made with the transfemoral Direct Flow Medical transcatheter aortic valve system in high-risk patients affected by severe aortic stenosis. Retrievability and repositionability of the prosthesis enhance control during deployment and may explain our findings, consisting in reduced aortic regurgitation, as well as high device success and safety rates. |
Conflict of interest statement
H. Ince, P. Frambach, S. Kische and P. den Heijer report receiving proctorship fees from Direct Flow Medical. A. Colombo is a minor shareholder in Direct Flow Medical. C. Naber is a consultant for Direct Flow Medical. A. Latib is a consultant and proctor for Direct Flow Medical. The other authors have no conflicts of interest to declare.

