Abstract
Background: TAVI is a widely accepted treatment for patients with severe aortic stenosis (AS). Despite the adoption of diverse therapies, opportunities remain to develop technologies tailored to provide optimal acute and potential long-term benefits, particularly around haemodynamics, flow and durability.
Aims: We aimed to evaluate the safety and feasibility of the DurAVR transcatheter heart valve (THV), a first-in-class biomimetic valve, in the treatment of patients with symptomatic severe AS.
Methods: This was a first-in-human (FIH), prospective, non-randomised, single-arm, single-centre study. Patients with severe, symptomatic AS of any surgical risk and who were eligible for the DurAVR THV prosthesis were recruited; they were assessed at baseline, 30 days, 6 months, and 1 year post-procedure for implant success, haemodynamic performance, and safety.
Results: Thirteen patients (73.9±6.4 years old, 77% female) were enrolled. The DurAVR THV was successfully implanted in 100% of cases with no device-related complications. One access site complication, one permanent pacemaker implantation, and one case of moderate aortic regurgitation occurred. Otherwise, no deaths, stroke, bleeding, reinterventions, or myocardial infarction were reported during any of the follow-up visits. Despite a mean annulus size of 22.95±1.09 mm, favourable haemodynamic results were observed at 30 days (effective orifice area [EOA] 2.00±0.17 cm2, and mean pressure gradient [MPG] 9.02±2.68 mmHg) and were sustained at 1 year (EOA 1.96±0.11 cm2, MPG 8.82±1.38 mmHg), resulting in zero patients with any degree of prosthesis-patient mismatch. Additionally, new valve performance measures derived from cardiovascular magnetic resonance displayed restoration of laminar flow, consistent with a predisease state, in conjunction with a mean coaptation length of 8.3±1.7 mm.
Conclusions: Preliminary results from the FIH study with DurAVR THV demonstrate a good safety profile with promising haemodynamic performance sustained at 1 year and restoration of near-normal flow dynamics. Further clinical investigation is warranted to evaluate how DurAVR THV may play a role in addressing the challenge of lifetime management in AS patients.
Introduction
Transcatheter aortic valve implantation (TAVI) has seen a significant evolution since it was first used in 2002, to today, where it has become an acceptable option for younger patients with lower surgical risk123. This transformation was primarily driven by the optimisation of the overall procedure, ease of use, size of delivery systems, reduction in residual paravalvular leak and the need for permanent pacemakers after implantation45. These incremental design advances have contributed to improved patient outcomes and the subsequent rapid adoption of TAVI globally567.
Despite these advances, achieving ideal haemodynamic performance with current TAVI devices remains a significant clinical concern89. Considerable opportunity exists to challenge the current paradigm of incremental design improvement, in pursuit of true valve design innovation, with the goals of restoring haemodynamic performance and exercise capability to a predisease state. Advanced multimodality imaging, particularly cardiovascular magnetic resonance (CMR), plays an increasingly important role in our understanding of aortic stenosis (AS) and in the clinical evaluation of haemodynamic parameters such as aortic flow patterns, which could become potential therapeutic targets as they may impact patient outcomes1011.
The DurAVR transcatheter heart valve (THV) (Anteris Technologies), a first-in-class biomimetic valve, is designed to mimic the performance of a healthy native aortic valve. Traditional bioprosthetic aortic valves comprise 3 separate pieces of flat tissue sutured together, which hinder a complete valve opening and, thus, result in smaller orifice areas and are potentially linked with abnormal flow patterns810. In contrast, the DurAVR THV is manufactured by moulding a single piece of tissue into the shape of a native aortic valve, facilitating a larger orifice area and near-normal flow patterns. This novel valve design, combined with the proprietary ADAPT (Anteris Technologies) tissue engineering anticalcification process, is mounted on a short-frame, balloon-expandable platform with large open-cell geometry for coronary access (Central illustration A). The objective of this first-in-human (FIH) study was to evaluate the safety and feasibility of the DurAVR THV in the treatment of patients with symptomatic severe AS and to better understand the differentiating impact of its unique biomimetic design.
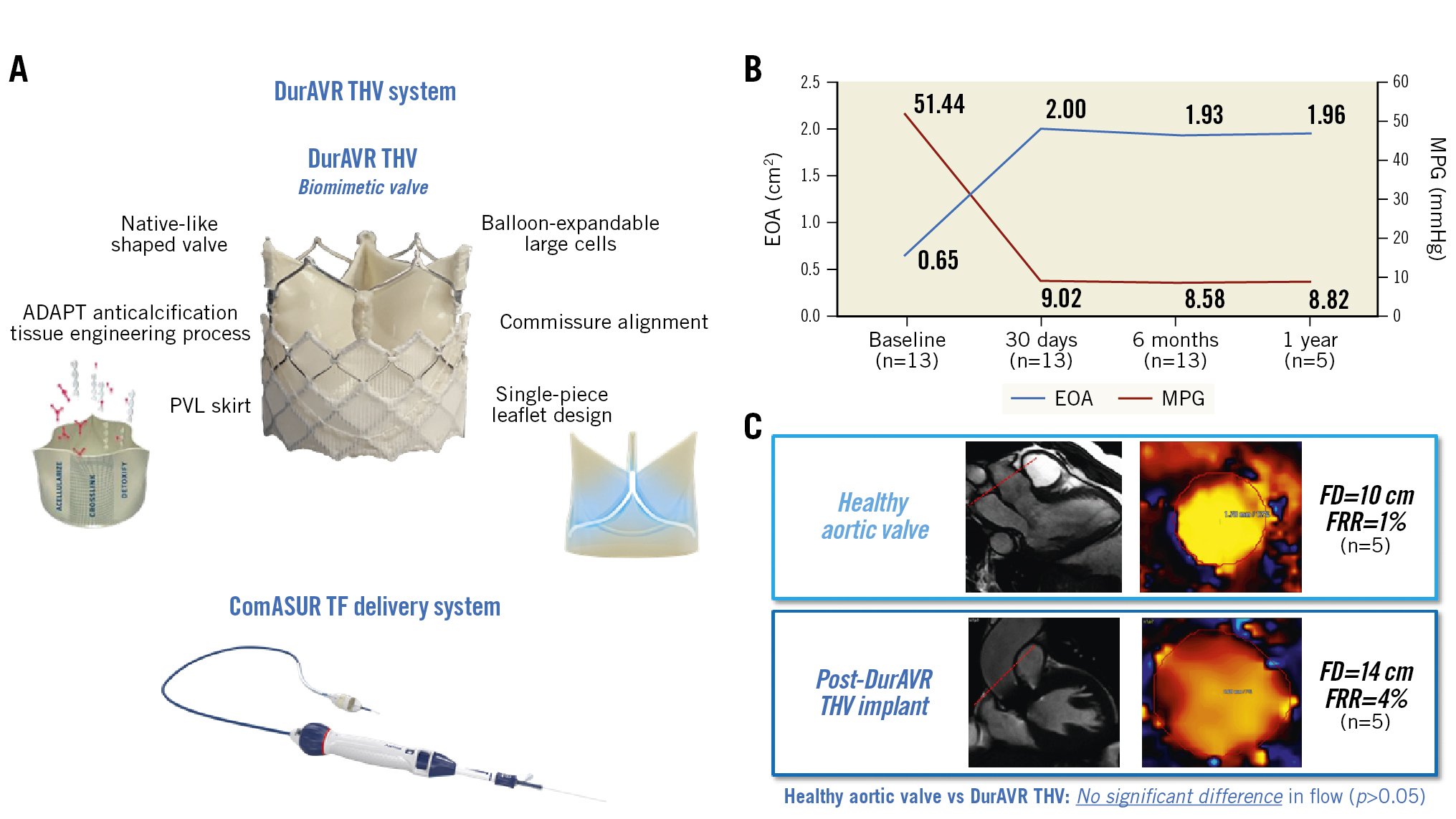
Central illustration. The DurAVR THV system: features, haemodynamic results and aortic flow characteristics. A) DurAVR THV system: DurAVR THV and ComASUR TF delivery system. B) Aortic valve haemodynamics: EOA and MPG at baseline, 30 days, 6 months, and 1 year post-procedure demonstrating favourable and sustained haemodynamic performance despite a mean annulus size of 22.95 mm and an aortic valve area of 409.22 mm2. C) Two-dimensional CMR at 6 months showing no significant difference in aortic flow characteristics between a healthy aortic valve and a DurAVR THV (p>0.05 for both FD and FRR), thus demonstrating restoration of laminar aortic flow post-DurAVR THV implantation. CMR: cardiac magnetic resonance; EOA: effective orifice area; FD: flow displacement; FRR: flow reversal ratio; MPG: mean pressure gradient; PVL: paravalvular leak; TF: transfemoral; THV: transcatheter heart valve
Methods
Study design
This was a prospective, single-arm, single-centre, FIH study (ClinicalTrials.gov: NCT05182307). The study protocol was approved by the local ethics committee of Tbilisi Heart and Vascular Clinic, Tbilisi, Georgia, and required informed consent of all subjects for treatment with an investigational device and confirmation of their participation in follow-up for one year.
Patient population
A total of 25 patients with severe, symptomatic AS of any surgical risk were screened for the study between November 2021 and May 2022. Of these, 12 patients were excluded leaving 13 patients in the study. Inclusion and exclusion criteria for participation in the study, in addition to screen failure rationale, are provided in Supplementary Appendix 1 and Supplementary Appendix 2. Eligibility of participants for delivery of the DurAVR THV prosthesis was determined by the study’s Heart Team. Healthy controls were recruited from Dr Pankaj Garg’s research study in Sheffield, UK, for a subanalysis. All participants gave informed consent for CMR imaging. This subanalysis was approved by the local ethics committee (18/NE/0186).
Device description
The Anteris DurAVR THV system is composed of the DurAVR THV and the ComASUR transfemoral (TF) delivery system (Anteris Technologies). The DurAVR THV comprises a balloon-expandable frame surrounding a single piece of bovine pericardial tissue moulded into a trileaflet valve to mimic the performance of a healthy native aortic valve. The bovine pericardium is treated with the proprietary ADAPT tissue engineering process. Compared to other biological materials used for cusp replacement, the ADAPT-treated bovine pericardium shows improved mechanical properties, like elasticity and strength, similar to healthy native aortic leaflets12. The single-piece structure aims at replicating the native valve tissue continuity and reduces the number of stitches needed in the manufacturing process, thus, leaving fewer potential sites for calcification initiation1314. The ADAPT tissue engineering process is a platform technology developed to mitigate bioprosthetic calcification, which impacts valve durability. It is based on a multifactorial approach where all known antigens (phospholipids, cells and cell remnants, nucleic acids, alpha-gal) and process chemistry (crosslinking and storage), responsible for the inflammatory response leading to calcification, are addressed15. The balloon-expandable frame features large, open-cell geometry and radiopaque markers to facilitate valve rotation and achieve commissural alignment to the native aortic valve for improved haemodynamic performance and coronary access. The valve also includes a polyethylene terephthalate (PET) skirt to reduce potential paravalvular leakage (PVL) (Central illustration A). The valve is currently available in 1 size and was used in our study for the treatment of native aortic annuli with an area-derived diameter of 21 to 25 mm. The DurAVR THV is directly crimped onto a balloon-expandable catheter and delivered transfemorally via the ComASUR TF delivery system.
Implant procedure
Given the FIH nature of this study, all procedures were performed using general anaesthesia and transoesophageal echocardiographic (TOE) guidance. For peripheral access cases, the ComASUR TF delivery system was used. A 22 Fr Gore DrySeal delivery sheath (W.L. Gore & Associates) − compatible with the expandable 14 Fr sheath currently in development − was introduced into the vasculature and a Safari wire (Boston Scientific) was placed in the left ventricle. Balloon aortic valvuloplasty (BAV) was performed with rapid ventricular pacing in all cases prior to valve implantation. The DurAVR THV was deployed under fluoroscopic guidance after assessment of its position in the proper anatomical location. Appropriate valve expansion, haemodynamic function, and the presence of paravalvular leaks and central aortic regurgitation were then assessed via TOE. European guidelines for anticoagulation management in valvular heart disease were followed, with all patients receiving low-dose acetylsalicylic acid (100 mg) post-TAVI or dual antiplatelet therapy, as needed6.
Study assessments and primary endpoints
Patients were assessed at baseline, and again at 30 days, 6 months, and 1 year post-procedure. Visits included medical, echocardiographic, cardiac computed tomography (CT) and CMR assessments, the Kansas City Cardiomyopathy Questionnaire (KCCQ) and a 6-minute walk test (6MWT). Primary performance endpoints were the correct positioning of a single DurAVR THV into the proper anatomical location and postoperative haemodynamic performance; safety endpoints were all-cause mortality, myocardial infarction, disabling stroke, and life-threatening bleeding at 30-day and 1-year follow-up. Study endpoints were defined according to the Valve Academic Research Consortium (VARC)-3 guidelines16.
Echocardiography
All echocardiograms were performed according to the American Society of Echocardiography guidelines for transthoracic echocardiogram (TTE) examination17. The grading of aortic stenosis was performed using mean gradients and peak velocities, as well as the dimensionless index. The effective orifice area (EOA) was calculated by the continuity equation18.
Cardiovascular magnetic resonance
CMR acquisition was performed on a 3.0T Ingenia (Philips). Balanced, steady-state, free precession, end-expiratory breath-hold cine images were acquired for the 2-, 3- and 4-chamber long-axis views and a stack of short-axis images, as per the standardised protocols19. Two-dimensional phase-contrast velocity-encoded flow with 30 frames per RR interval was acquired in the ascending aorta at an orthogonal plane just above the sinotubular junction and at the mid-level of the main pulmonary artery.
Cardiac magnetic resonance image analysis
All CMR image analyses were performed at a core laboratory using commercial research software MASS (version 2022 EXP; Leiden University Medical Center). Peak systolic flow displacement (FD), a marker of flow eccentricity, was calculated as the distance between the vessel centreline and the centre of the eccentric flow. It was normalised for overall vessel size at the peak systolic phase11 and is presented as a percentage in this paper. Peak systolic flow reversal ratio (FRR) was calculated as previously described in the literature20.
Statistical analysis
This is a FIH feasibility study, and thus, primary endpoints are not powered for statistical differences to historical controls. The sample size allowed investigators to make a qualitative assessment of the safety of DurAVR THV in the population studied. Study endpoints, including baseline characteristics, procedural details and clinical outcomes, are presented as percentage for categorical variables and mean±standard deviation for continuous variables without formal statistical testing.
Results
Baseline patient characteristics
A total of 13 patients were enrolled in the study. Baseline characteristics of the intention-to-treat (ITT) population are provided in Table 1. The mean age was 73.9±6.4 years, and 10 (77%) were female. Overall, patients had a mean Society of Thoracic Surgeons (STS) score of 2.3±1.1%. Prior to procedures, 11 patients (85%) were in New York Heart Association (NYHA) Functional Class II, and 2 (15%) were in NYHA Class III. Additionally, the mean aortic annulus systolic diameter by computed tomography angiography (CTA) was 22.95±1.09 mm. Several challenging anatomies were treated in this FIH study, including patients with severe annular calcium, extreme leaflet calcium, type 1 bicuspid aortic valve morphology, and severe left ventricular outflow tract (LVOT) calcium.
Table 1. Patient baseline characteristics.
| Characteristics | n=13 | |
|---|---|---|
| Age, years | 73.9±6.4 | |
| Gender, female | 10 (77) | |
| Society of Thoracic Surgeons score, % | 2.3±1.1 | |
| EuroSCORE II, % | 1.5±0.7 | |
| New York Heart Association Functional Class | I | 0 (0) |
| II | 11 (85) | |
| III | 2 (15) | |
| IV | 0 (0) | |
| Medical history and previous cardiovascular interventions | Coronary artery disease | 9 (69) |
| Aortic valve insufficiency (mild to moderate) | 9 (69) | |
| History of conduction disturbances | 6 (46) | |
| Mitral valve insufficiency | 5 (38) | |
| Smoker (former or current) | 4 (31) | |
| Previous myocardial infarction | 3 (23) | |
| Diabetes | 3 (23) | |
| Tricuspid valve insufficiency | 3 (23) | |
| Obesity | 2 (15) | |
| Cerebrovascular disease | 0 (0) | |
| Chronic obstructive pulmonary disease | 0 (0) | |
| Percutaneous coronary intervention | 8 (62) | |
| Coronary artery bypass grafting | 2 (15) | |
| Permanent pacemaker implant | 1 (8) | |
| Computed tomography data | ||
| Aortic root analysis | Left ventricular outflow tract diameter, mm | 23.18±1.81 [20.7, 26.6] |
| Annulus valve analysis | Perimeter, mm | 72.58±3.49 [67.9, 78.4] |
| Perimeter-derived diameter, mm | 23.09±1.11 [21.6, 24.9] | |
| Aortic area, mm2 | 409.22±38.47 [355.2, 479.6] | |
| Area-derived diameter, mm | 22.95±1.09 [21.3, 24.5] | |
| Minimum diameter, mm | 20.54±1.13 [17.8, 22.7] | |
| Maximum diameter, mm | 25.38±1.34 [23.5, 28] | |
| Eccentricity | 0.19±0.05 [0.14, 0.28] | |
| Sinus of Valsalva diameter | Left, mm | 30.50±1.18 [28.5, 33.1] |
| Right, mm | 29.26±1.71 [26.4, 33.0] | |
| Non-coronary, mm | 30.85±2.36 [27.3, 35.2] | |
| Height of coronary ostia | Left, mm | 13.99±2.68 [9.1, 19.1] |
| Right, mm | 18.06±3.49 [12.1, 23.5] | |
| Sinotubular junction diameter, mm | 27.81±2.74 [23.9, 32.7] | |
| Sinotubular junction height, mm | 20.00±1.79 [18.5, 24.3] | |
| Ascending aorta diameter, mm | 34.41±4.53 [27.4, 42.3] | |
| Data are presented as n (%) or mean±standard deviation [min, max]. EuroSCORE: European System for Cardiac Operative Risk Evaluation | ||
Procedural characteristics and clinical endpoints
A single DurAVR THV was successfully implanted in each of the 13 patients enrolled in the study (100% success). Procedural characteristics and details are shown in Table 2. The first cohort of 5 patients enrolled in November 2021 underwent a TAVI procedure with a direct aortic approach. The second cohort of 8 patients was enrolled in May 2022 after development of the ComASUR TF delivery system. The vascular access was transfemoral in 7 cases and transcarotid in 1 case. There were no device-related complications, need for a second TAVI device, nor conversions to open heart surgery. One access site complication (thrombosis of the external iliac artery and common femoral artery secondary to the access site closure) occurred. The patient underwent vascular surgery on postoperative day 1 and the event was resolved with a favourable outcome. In addition, 1 new permanent pacemaker was implanted as a precautionary measure on postoperative day 6 in a patient with pre-existing right bundle branch block (QRS duration 144 ms prior to DurAVR THV implant) and left anterior fascicular block.
The study safety endpoints are provided in Table 3. No deaths, myocardial infarction, stroke, minor or life-threatening bleeding occurred during any of the follow-up timepoints of 30 days, 6 months and 1 year. There were no device-related complications, reoperation or reinterventions reported or changes in QRS and PR intervals observed throughout follow-up, apart from the patient who was implanted with the permanent pacemaker. Additionally, no neurological dysfunction or acute kidney injury were recorded. Outcomes for the 6MWT showed improvement from baseline with a 31% increase in the average walked distance at 1 year. Moreover, the KCCQ score was 20.4 points higher at 6 months (n=13) and 22.9 points higher at 1-year (n=5) follow-up compared to baseline, demonstrating continuous improvement in exercise capacity and quality of life.
Echocardiography results demonstrate a robust and consistent haemodynamic performance up to 1-year follow-up (Table 4). Despite a small mean annulus diameter (22.95±1.09 mm on baseline CT), TTE at 30 days post-procedure showed a mean EOA of 2.00±0.17 cm2, mean indexed effective orifice area (iEOA) of 1.14±0.15 cm2/m2, mean pressure gradient (MPG) of 9.02±2.68 mmHg, and mean Doppler velocity index (DVI) of 0.53±0.10. The echo assessment at 6 months (n=13) confirmed sustained performance with a mean EOA of 1.93±0.15 cm2 (mean iEOA 1.10±0.18 cm2/m2), mean MPG of 8.58±2.04 mmHg and mean DVI of 0.55±0.10. The one-year results on the first five patients reported a mean EOA of 1.96±0.11 cm2 (mean iEOA 1.11±0.07 cm2/m2), mean MPG of 8.82±1.38 mmHg, and mean DVI of 0.54±0.05, further strengthening these findings (Central illustration B). In addition, no moderate or severe prothesis-patient mismatch (PPM) was observed (Table 4)21.
There was one case of moderate central aortic regurgitation (AR) secondary to balloon overexpansion of the proximal valve frame in the first case of the second patient cohort (immediately addressed prior to the following cases) that remained unchanged at 6-month follow-up. For the entire cohort, at 30 days, 6 months and 1 year, there was no moderate or severe PVL. At 30 days, 5 cases had none/trace PVL, while at 6 months, PVL was none/trace in 7 cases. For the first 5 patients who completed their 1-year assessment, 2 had mild PVL and 3 had none/trace.
The postprocedural CT results at 30 days confirmed normal leaflet mobility and no calcifications. CT images showed the DurAVR THV in the same position as at implant and no frame deformation. There was one case of hypoattenuated leaflet thickening (HALT) limited to the base and one case of 50-75% HALT on the right cusp. None of these subclinical findings led to changes in haemodynamics and/or symptoms. Both patients started oral anticoagulation therapy, which resulted in the complete resolution of the 50-75% HALT, while the other remained unchanged.
Table 2. Procedural characteristics.
| Parameters | n=13 | |
|---|---|---|
| Approach | Transfemoral | 7 (54) |
| Transaortic | 5 (38) | |
| Transcarotid | 1 (8) | |
| General anaesthesia | 13 (100) | |
| Predilatation | 13 (100) | |
| Post-dilatation | 10 (77) | |
| Contrast media, ml | 105±32 | |
| Fluoroscopy time, mm:ss | 19:54±06:24 [09:19, 33:01] | |
| DurAVR THV implant time, hh:mm | 0:18±0:10 [0:07; 0:46] | |
| DurAVR THV implant success* | 13 (100) | |
| Coaptation length (intraprocedural TOE), mm | 8.3±1.8 [6.0, 11.1] | |
| Postoperative transthoracic echocardiography | ||
| Mean pressure gradient, mmHg | 8.34±1.74 [6.00, 11.00] | |
| Effective orifice area, cm2 | 2.12±0.29 [1.83, 2.71] | |
| Major paravalvular leak (moderate or severe) | 0 (0) | |
| Major aortic regurgitation (moderate or severe) | 0 (0) | |
| Data are presented as n (%) or mean±SD [min, max]. *One single DurAVR THV implanted with no device-related complications nor conversion to open heart surgery. SD: standard deviation; THV: transcatheter heart valve; TOE: transoesophageal echocardiography | ||
Table 3. Cumulative events at 30-day, 6-month and 1-year follow-up.
| Event | 30-day follow-up n=13 | 6-month follow-up n=13 | 1-year follow-upn=5 |
|---|---|---|---|
| Primary safety endpoints | |||
| All-cause mortality | 0 (0) | 0 (0) | 0 (0) |
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) |
| Disabling stroke | 0 (0) | 0 (0) | 0 (0) |
| Life-threatening bleeding | 0 (0) | 0 (0) | 0 (0) |
| VARC-3 endpoints* | |||
| Valve-related mortality | 0 (0) | 0 (0) | 0 (0) |
| All strokes | 0 (0) | 0 (0) | 0 (0) |
| Neurological dysfunctions | 0 (0) | 0 (0) | 0 (0) |
| All bleeding | 0 (0) | 0 (0) | 0 (0) |
| Major vascular complications | 1 (7.69) | 0 (0) | 0 (0) |
| Minor vascular complications | 0 (0) | 0 (0) | 0 (0) |
| Cardiac complications | 0 (0) | 0 (0) | 0 (0) |
| Permanent pacemaker implant | 1 (7.69) | 0 (0) | 0 (0) |
| Bioprosthetic valve dysfunction | 0 (0) | 0 (0) | 0 (0) |
| Structural valve deterioration | 0 (0) | 0 (0) | 0 (0) |
| Reoperations/reinterventions on the study valve | 0 (0) | 0 (0) | 0 (0) |
| Acute kidney injury | 0 (0) | 0 (0) | 0 (0) |
| Major paravalvular leak (moderate or severe) | 0 (0) | 0 (0) | 0 (0) |
| Major aortic regurgitation (moderate or severe) | 1 (7.69) | 1 (7.69) | 0 (0) |
| Values are n (%). *Study endpoints are defined according to the VARC-3 guidelines16. VARC: Valve Academic Research Consortium | |||
Table 4. Clinical and haemodynamic results at baseline, 30-day, 6-month and 1-year follow-up.
| Baseline | 30 days | 6 months | 1 year | |
|---|---|---|---|---|
| NYHA Class | n=13 | n=13 | n=13 | n=5 |
| NYHA I | 0 (0) | 2 (15.4) | 4 (30.8) | 2 (40.0) |
| NYHA II | 11 (84.6) | 9 (69.2) | 9 (69.2) | 3 (60.0) |
| NYHA III | 2 (15.4) | 2 (15.4) | 0 (0) | 0 (0) |
| NYHA IV | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 6-minute walk test (6MWT) | n=13 | n=13 | n=12* | n=4* |
| Total walked distance, m | 230.9±45.7 | 278.2±59.3 | 306.0±52.8 | 323.5±48.1 |
| KCCQ | n=13 | n=13 | n=13 | n=5 |
| Overall summary score | 37.8±8.4 | 52.8±9.7 | 58.2±14.1 | 67.8±18.8 |
| Electrocardiogram results | n=13 | n=13 | n=13 | n=5 |
| PR interval, ms | 172.7±44.9 | 176.6±41.5 | 184.6±50.5 | 184.4±46.0 |
| QRS interval, ms | 107.2±23.9 | 107.5±22.0 | 111.5±25.2 | 108.4±24.3 |
| Haemodynamic results | n=13 | n=13 | n=13 | n=5 |
| Effective orifice area, cm2 | 0.65±0.20 | 2.00±0.20 | 1.93±0.12 | 1.96±0.10 |
| Indexed effective orifice area , cm2/m2 | 0.37±0.10 | 1.14±0.20 | 1.10±0.20 | 1.11±0.10 |
| Mean pressure gradient, mmHg | 51.44±18.3 | 9.02±2.7 | 8.58±2.0 | 8.82±1.4 |
| Peak pressure gradient, mmHg | 85.48±29.9 | 18.66±5.67 | 16.58±4.3 | 18.06±3.3 |
| Doppler velocity index | 0.20±0.00 | 0.53±0.10 | 0.55±0.10 | 0.54±0.10 |
| Left ventricular ejection fraction, % | 57.0±7.3 | 59.0±11.5 | 59.0±12.0 | 56.0±9.5 |
| Moderate prosthesis-patient mismatch | N/A | 0 (0) | 0 (0) | 0 (0) |
| Severe prosthesis-patient mismatch | N/A | 0 (0) | 0 (0) | 0 (0) |
| Data are presented as n (%) or mean±SD. *One patient unable to complete the 6MWT at 6 months and 1 year due to hip replacement. KCCQ: Kansas City Cardiomyopathy Questionnaire; N/A: not applicable; NYHA: New York Heart Association; SD: standard deviation | ||||
New parameters for performance assessment of the DurAVR THV biomimetic valve
The unique leaflet design and performance of DurAVR THV was evaluated in multiple modalities. Intraoperative TOEs revealed a mean leaflet coaptation length of 8.3±1.8 mm; CT scan results at 30 days were consistent with these findings and visually displayed the unique leaflet design in vivo (Figure 1A).
The TTE at 30 days displayed consistent laminar flow throughout the valve (Figure 1B). In addition, the first 5 patients underwent CMR which incorporated two-dimensional phase contrast at the level of the ascending aorta at 6 months to investigate the aortic flow physiology post-DurAVR THV implantation. Aortic flow characteristics were assessed through the measurement of aortic FD and aortic systolic FRR. The average FD was 14±10 cm while the average FRR was 4±6%. The results of the first 5 patients who received the DurAVR THV were compared with those of 5 age/height/weight-matched controls with healthy native aortic valves (Central illustration C). Characteristics of the healthy controls and their comparison to the DurAVR THV group are provided in Supplementary Appendix 3. DurAVR THV recipients had comparable flow displacement (14±10 cm vs 10±5 cm; p=0.453) and minimal flow reversal ratio (4±6% vs 1±1%; p=0.328) to the controls. Together these results, in conjunction with the echocardiographic findings at 30 days, indicate that the novel leaflet design of DurAVR THV restores laminar aortic flow.
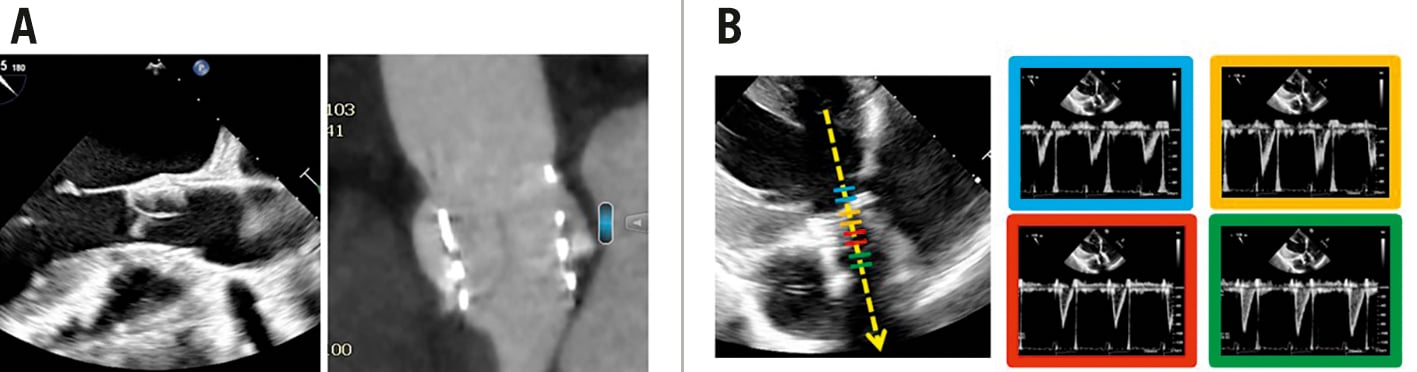
Figure 1. DurAVR THV is the first AVR shown to restore normal aortic blood flow. A) Intraoperative TOE (left) and 30-day CT scan (right) displaying the DurAVR THV leaflet coaptation length. B) 30-day TTE, showing consistent laminar flow throughout the valve. AVR: aortic valve replacement; CT: computed tomography; TOE: transoesophageal echocardiography; THV: transcatheter heart valve; TTE: transthoracic echocardiography
Discussion
In this study we report the first clinical use of the novel biomimetic DurAVR THV. High implant success with a good safety profile was demonstrated. In this small sample size, none of the safety endpoints (mortality for any cause, myocardial infarction, disabling stroke, and life-threatening bleeding) occurred. One case of permanent pacemaker implantation (PPI) was reported in a patient with pre-existing conduction disturbances. Of note, no increase in the QRS interval from baseline was observed in the remaining patients throughout the study. The 30-day TTE showed 1 case of moderate central AR secondary to balloon overexpansion that remained unchanged up to the 6-month follow-up. Considering this is an FIH study, we chose to perform pre-BAV in all cases and elected to be conservative in our implant strategy, undersizing at first and then adding volume. In 77% of the cases, we also performed post-dilatation. Moving to larger clinical studies, we expect lower pre- and post-dilation rates, similar to other balloon-expandable THVs22.
The DurAVR THV displayed an excellent haemodynamic performance up to 1-year follow-up despite a small mean patient annulus size. Contemporary data from clinical studies with commercially available TAVI technologies in the US showed a smaller EOA in patients with a similar baseline native aortic valve area or native annular diameter23. While DurAVR THV showed a mean EOA of 2.00±0.17 cm2 at 30 days, in a study by Hahn et al23, a mean EOA of 1.58±0.33 cm2 was reported for the SAPIEN 3 (Edwards Lifesciences) group (native aortic valve area 385-439 mm2) and a mean EOA of 1.82±0.43 cm2 was reported for the Evolut R (Medtronic) group (native annular diameter >22.3 to ≤23.3 mm). We believe the favourable haemodynamic findings in our study are the result of the biomimetic design of DurAVR THV and the long coaptation length, which allow for a larger EOA.
The restoration of near-normal haemodynamics likely contributed to improved exercise capacity, measured by the 6MWT, and improved quality of life, assessed through the KCCQ. This is particularly relevant with the current change in guidelines, with TAVI now recommended in younger and low-risk patients who are expected to have an active lifestyle67.
Previous observational studies using four-dimensional (4D) flow CMR to quantify aortic flow haemodynamics have demonstrated that TAVI devices have a greater degree of flow eccentricity than surgical aortic valve replacement (SAVR)10, potentially leading to increased wall shear stress, arterial stiffness, and aortic root dilatation2425, which is an undesirable clinical outcome if TAVI was to be considered in younger cohort of patients. More recently, a large observational study also confirmed that systolic flow reversal is independently associated with ascending aorta dilatation even without any significant gradient across the aortic valve26. Our CMR substudy reported for the first time restoration of aortic flow eccentricity and systolic flow reversal ratio. Collectively, our data indicate that the novel leaflet design of the DurAVR THV restores ascending aortic flow patterns. This physiological advantage may have potential clinical benefits in reducing the risk of aortic dilatation and remodelling and ameliorating the potential increased aortic stiffness seen after SAVR27. Additional studies are warranted to investigate the prognostic importance of restoration of normal aortic flow on durability, myocardial recovery, and myocardial remodelling.
Study limitations
This study is not without limitations, given this is a first-in-human experience, with a small sample size and no comparator arm. Thus, the current study provides insights for future trials with larger populations and adequate power to validate clinical outcomes. Moreover, this study was not statistically powered for clinical endpoints at each follow-up timepoint and, therefore, results should be interpreted with caution and considered hypothesis-generating only. Given the length of follow-up, the study was not intended to prove the durability of the DurAVR THV System.
Conclusions
The DurAVR THV FIH study provides encouraging echo and CMR evidence of near-normal haemodynamics and normalised flow characteristics associated with its unique native-like properties at 1-year follow-up. The preliminary observations provided here will serve as a basis for future powered clinical trials to gain a better understanding of how the acute benefits of a biomimetic design (haemodynamics and normalised flow) impact on quality of life, durability, myocardial recovery, myocardial remodelling and aortopathies in the long-term treatment of AS.
Impact on daily practice
At 1-year follow-up, the DurAVR THV FIH study provides encouraging echo and CMR evidence of improved haemodynamics and normalised flow characteristics associated with its unique single-piece leaflet design. These findings demonstrate that valve leaflet design appears to play a significant role in acute haemodynamic benefits (EOA and MPG), as well as aortic flow patterns (laminar flow), leading to potential short- and long-term favourable clinical implications. Additional studies and further direct comparisons with other commercial TAVI systems will establish the clinical value of this new and highly differentiated TAVI device.
Acknowledgements
JetPub Scientific Communications LLC assisted the authors in the preparation of this manuscript. Jason Quill (Anteris Technologies) contributed to the development of the DurAVR THV system and supported the authors during its implantation. Ioana Ghiu and Silvia Zinicchino (Anteris Technologies) supported the conduction of this FIH study and assisted the authors in the preparation of this manuscript.
Funding
This study was sponsored by Anteris Technologies, Eagan, MN, USA.
Conflict of interest statement
S.K. Kodali is a consultant for Anteris Technologies, TRiCares, TriFlo Cardiovascular, xDot Medical, Micro Interventional Devices, Supira Medical, Adona Medical, Tioga Cardiovascular, HVR Cardio, and Moray Medical; serves on the scientific advisory boards for Dura Biotech, Thubrikar Aortic Valve, Philips, Medtronic, Boston Scientific, and Abbott Laboratories; and has received institutional research funding from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve Technology. P. Sorajja serves on the advisory boards of 4C Medical Technologies, Abbott Structural (Global Valve Masters, Global Complexity Score, Tricuspid Advisory Board), Boston Scientific SHV Strategic Advisory Board, Medtronic Structural Advisory Board, and VDyne; has received consulting fees from 4C Medical Technologies, Anteris Technologies, Abbott Structural, Boston Scientific, Edwards Lifesciences, Evolution Medical Technologies, Foldax, GLG, Medtronic, Philips, Siemens, Shifamed, W.L. Gore, VDyne, and xDot Medical; and served as member of REPAIR MR Steering Committee, EXPAND Steering Committee, W.L. Gore Pipeline Executive Committee, SUMMIT Steering Committee, TRILUMINATE Steering Committee, TRILUMINATE Eligibility Committee for Anatomy, G4 Adverse Leaflet Events Committee, Neovasc Executive Steering Committee; National or Global Principal Investigator for TRILUMINATE Pivotal US Trial, SUMMIT MAC Pivotal Trial, EXPAND EFS and Pivotal US Trial, HighLife (USA) EFS, VDyne EFS, SOAR EFS and AMEND EFS. C.U. Medori is the CMO at Anteris Technologies; received grants/research support from Boston Scientific; and received honoraria/consultation fees from Abbott, Alleviant Medical, Boston Scientific, Cardiovalve, VDyne, and xDot Medical. K. Feldt has received consulting fees from Anteris Technologies, and Alleviant Medical; payment or honoraria from AstraZeneca, Pfizer, and Abbott; and participates on an advisory board at Amgen. J.L. Cavalcante has served on advisory boards for Abbott Structural, Boston Scientific, and Medtronic; received consulting fees from 4C Medical Technologies, Anteris Technologies, Abbott Structural, Aria CV, Boston Scientific, Edwards Lifesciences, JenaValve Technology, Medtronic, VDyne, W.L. Gore, and XyloCor; and received research/grant support from Abbott Northwestern Hospital Foundation, and Abbott Structural. P. Garg serves on the advisory boards for Pie Medical Imaging, and Medis Medical Imaging; has received consulting fees from Anteris Technologies, and Edwards Lifesciences; is part of the Executive Committee for the British Society of Cardiovascular Magnetic Resonance; and is principal investigator for the PREFER-CMR trial. N. Hamid received consulting fees from Anteris Technologies, Edwards Lifesciences, Philips, Alleviant Medical, AMX Medical Systems, Axis Medical, 4C Medical Technologies, Valcare Medical, VDyne, and W.L. Gore. K.K. Poon has received grants from Edwards Lifesciences and Abbott Structural; and honoraria from Edwards Lifesciences and Abbott Structural. M. Settergren received grants/research support from Boston Scientific, W.L. Gore, and Abbott Vascular; honoraria/consultation fees from Anteris Technologies, Abbott Vascular, Boston Scientific, Edwards Lifesciences, CardioMech, Teleflex, The Shape Sensing Company, SmartCella, Segulah Medical, and Protembis. M.R. Burns declares consulting fees from Anteris Technologies, W.L. Gore, Edwards Lifesciences, and VDyne. A. Rück received support from Anteris Technologies; grants from Boston Scientific; consulting fees from Boston Scientific and Edwards Lifesciences; honoraria from Boston Scientific, Edwards Lifesciences, and Abbott; and served on an advisory board for Boston Scientific. J. Sathananthan received consulting fees from Edwards Lifesciences, Medtronic, Boston Scientific, and Anteris Technologies; and research funding from Edwards Lifesciences and Medtronic. A. Zajarias declares consulting fees from Edwards Lifesciences, Medtronic, and Anteris Technologies; and serves on an advisory board for Medtronic. V. Bapat received consulting fees from Anteris Technologies, Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

