
Introduction
The usefulness of revascularisation strategies for renal artery stenosis is the subject of debate following the publication of trials showing that percutaneous revascularisation is not superior to medical therapy alone1,2. Haemodynamic measurements of renal artery stenosis may help to identify better those patients suitable for revascularisation3. Renal flow reserve (RFR), the relative increase in blood flow velocity after maximal vasodilatation, may offer pivotal additional information on renal vascular reactivity and function and may help in selecting patients who may benefit from revascularisation. We studied the feasibility of intrarenal pressure and flow velocity measurements and examined the intra-individual reproducibility of RFR.
Methods
Between March 2014 and December 2017, we included patients scheduled for elective coronary or renal angiography. All patients provided written informed consent. A 0.014-inch dual pressure and Doppler sensor-equipped guidewire (ComboWire®; Philips Volcano, San Diego, CA, USA) was used to measure renal flow velocity and distal renal pressure simultaneously. Study procedures are outlined in Supplementary Figure 1. Proximal (aortic) pressure was measured by a fluid-filled line in the guiding catheter. Hyperaemia was induced by injection of dopamine 30 µg/kg over a time span of 60 seconds directly into the renal artery. In between measurements, a waiting period of at least 10 minutes was maintained starting from the decrease in renal flow velocity. Then, the wire was repositioned in the index renal artery and the measurements were repeated. RFR was defined as the ratio of hyperaemic to resting averaged peak Doppler flow velocity (APV).
SAMPLE SIZE CALCULATION
Based on the results reported by Manoharan et al4, we calculated that we would need a total of 23 patients in order to detect a 25% difference between RFR measurements at a two-sided significance level of 0.05 with 80% power, assuming a standard deviation of 0.6.
Results
We included 34 patients to obtain 23 successful repeated measurements. The total success rate of intrarenal measurements was 82%, with at least one successful RFR measurement in 28 out of 34 subjects. Patient characteristics were not different to those in individuals with unsuccessful measurements (Supplementary Table 1). An example of pressure and flow velocity measurements in a stenosed renal artery is shown in Figure 1.
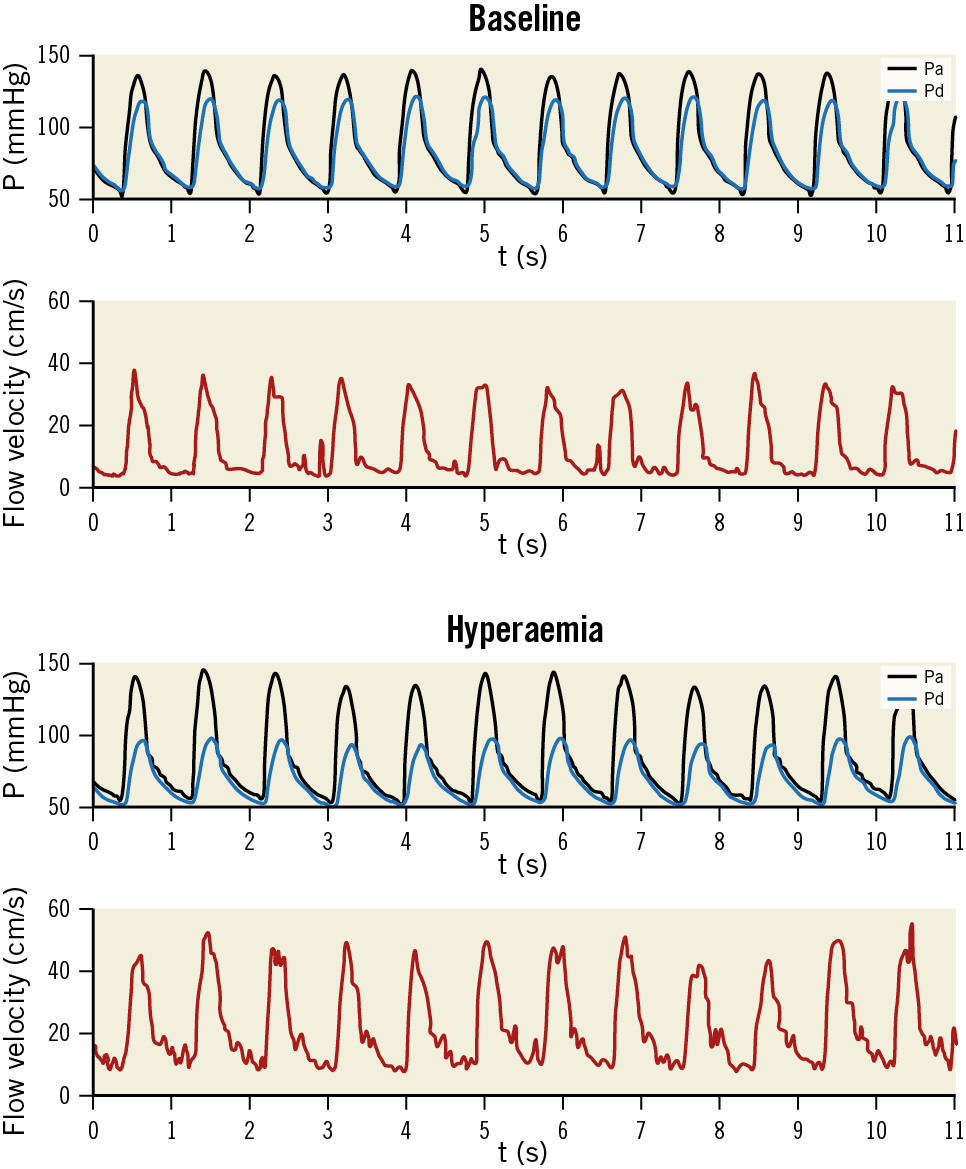
Figure 1. Example of pressure and flow velocity measurements in a stenosed renal artery. Black lines: aortic pressure (Pa). Blue lines: distal pressure (Pd). Red lines: flow velocity. Upper two panels depict measurements during baseline conditions, lower two panels represent hyperaemic measurements.
In those with successful repeated measurements, the average age was 58 years, interquartile range (IQR) 52-64, 16 (70%) were male, and mean body mass index (BMI) was 27 kg/m2 (IQR: 24-31). Eight patients (35%) had a history of diabetes mellitus and 16 (70%) had a history of cardiovascular disease. Twelve patients (52%) used an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.
The mean RFR was 2.16 in measurement 1 and 2.21 in measurement 2 (p=0.56) (Supplementary Figure 2), with a corresponding intraclass correlation coefficient of 0.82 (95% CI: 0.63–0.92; p<0.001). The coefficient of variation for RFR was 36.8% for measurement 1 and 31.0% for measurement 2 (p=0.33). Figure 2 shows the flow velocities and central mean aortic blood pressures of the individual measurements (delta lines) and the median and interquartile values (box plots) at baseline and during hyperaemia. Mean arterial pressure decreased during hyperaemia, but to a similar extent (−7.2±7.8 mmHg [p<0.01] vs −5.9±5.0 mmHg [p<0.01]; p=0.52 for comparison of means) (Supplementary Table 2). APV prior to the second dopamine infusion was higher than baseline APV (p=0.03), whereas maximal APV during hyperaemia (APVmax) did not differ between measurements (p=0.83). Bland-Altman analysis revealed no systematic or proportional bias (mean difference −0.16 and t-score −1.19; p=0.25) (Supplementary Figure 3).
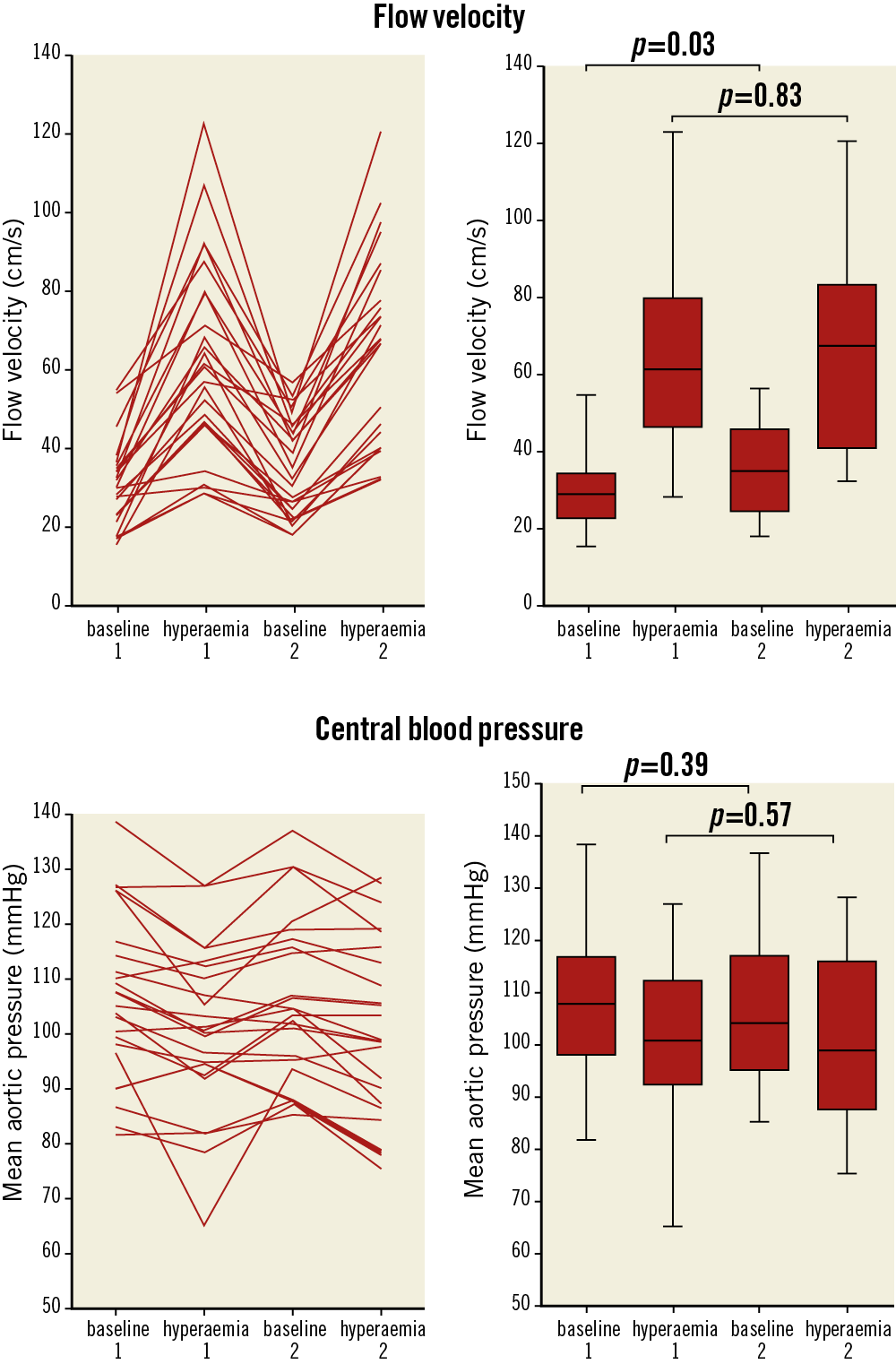
Figure 2. Delta lines and box plots depicting the medians and interquartile range of flow velocity and aortic pressures during baseline and hyperaemia in measurements 1 and 2.
Stratification according to median RFR showed that more subjects with a history of diabetes mellitus had a low RFR (8 versus 1 subjects; p<0.01) (Supplementary Table 3). Other baseline characteristics, including use of vasoactive medication, were evenly distributed.
Discussion
The fact that maximal responses were comparable despite residual effects (i.e., higher APV) during the second measurement further suggests that a plateau phase was reached and confirms previous findings that maximal hyperaemia is achieved with intrarenal dopamine 30 µg/kg4. The excellent reproducibility of the RFR measurements shows that possible variations in proximal vessel diameters had little impact on the hyperaemic measurements. Interestingly, almost all patients with a history of diabetes mellitus had lower than median RFRs. Although the study was not designed to link clinical features to RFR, this at least supports the findings in cardiology, where diabetes has been linked to impaired coronary flow reserve and microvascular disease5.
The investigators identified three manoeuvres that resulted in notable improvements in attaining the best signals. First, curling the tip of the measurement wire backwards appeared to be of great value, with the understanding that the position of the wire was secured against the vessel wall. Second, relocating the tip of the wire past the first bifurcation was helpful when no stable signal was found in the main branch of the renal artery. Third, operators noted that measurements performed via the femoral artery rather than the radial artery resulted in a more stable signal, probably because of diminished interference of thoracic breathing motions combined with a relatively long route to reach the renal artery’s orifice.
Limitations
The measurements were performed in patients with and without renal artery stenosis, although most patients had normal renal arteries and only a few had haemodynamically significant renal artery stenosis. We further observed a small decrease in central blood pressure and an increase in heart rate after dopamine infusion. Although these changes were significant, the haemodynamic changes had little influence on the measurements of RFR.
Conclusion
We show that combined intrarenal pressure and flow velocity measurements are feasible and that measurement of RFR following dopamine is reproducible. A single bolus administration of dopamine 30 µg/kg appeared to be sufficient for the induction of maximal hyperaemia, given the fact that a plateau phase was reached.
|
Impact on daily practice RFR has the potential to be a useful asset to renal fractional flow reserve (rFFR) in predicting the outcome of percutaneous renal angioplasty, especially in light of previous clinical studies that have not been able to show that rFFR alone is sufficient to predict treatment effects3. A two-dimensional approach using rFFR and RFR provides information on both proximal and distal vascular function and, similar to measurements in the coronary circulation, may contribute to clinical decision making and optimisation of treatment in patients with renal artery stenosis3. Future studies should focus on testing the predictive power of RFR and rFFR measurements on treatment outcome of renal revascularisation. |
Funding
This study was funded by the medical departments involved.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

