Abstract
Aims: The aims of this study were to evaluate the effects of renal stenting on cardiac function using echocardiographic parameters, and to clarify whether changes in clinical and echocardiographic variables after renal stenting differ between atherosclerotic renal artery stenosis (ARAS) patients with and without cardiac symptoms.
Methods and results: A total of 61 patients who underwent renal stenting and echocardiography were included in the study. Left ventricular (LV) filling pressure and LV relaxation were evaluated with tissue Doppler imaging. The ratio of the peak early diastolic mitral inflow velocity to the peak early mitral annular velocity (E/e’ ratio) and the e’-velocity were measured to assess diastolic function. LV ejection fraction remained unchanged, but the E/e’ ratio (P<0.001) and the e’-velocity (P=0.004) improved after renal stenting. In particular, the E/e’ ratio improved from 13.7±5.6 to 11.9±4.0 (P=0.002) within 24 hours after renal stenting and remained low at 11.2±3.8 after a mean follow-up period of 7±4 months (P=0.001). Patients with cardiac symptoms showed significantly better change in E/e’ ratio (P=0.002) and E-velocity (P=0.005) compared to those without cardiac symptoms. Cardiac symptoms also significantly improved after renal stenting (New York Heart Association functional class: 2.5±0.6 at baseline to 1.4±0.6 at follow-up; P<0.001).
Conclusions: Renal stenting improved echocardiographic parameters that reflect LV diastolic function, and yielded a higher benefit for E/e’ ratio and E-velocity in patients with cardiac symptoms than in those without cardiac symptoms.
Introduction
Atherosclerotic renal artery stenosis (ARAS) is a progressive disorder that blocks blood flow to the kidneys1,2. Such blood flow constitutes approximately one-fifth of the blood pumped by the heart under normal conditions. Grüntzig et al3 and Mahler et al4 first reported successful balloon angioplasty of the renal artery over three decades ago. Subsequently, the favourable effects of renal intervention on blood pressure and renal function have produced wider acceptance of renal stenting5-7, with current focus on improvement of cardiac destabilisation syndrome after the procedure8. The aims of the present study were 1) to evaluate the effects of renal stenting on cardiac function using echocardiographic parameters, and 2) to clarify whether changes in clinical and echocardiographic variables after renal stenting differ between ARAS patients with and without cardiac symptoms.
Methods
Study population
A total of 61 consecutive patients treated by a single operator were enrolled in the study. All the subjects underwent an echocardiographic examination including tissue Doppler imaging (TDI) before and after renal stenting. The study was approved by an institutional review committee and the subjects gave informed consent. Patients with ARAS undergoing renal stenting satisfied one or more of the following clinical indications for revascularisation: 1) suboptimal control of hypertension by at least two antihypertensive agents, 2) renal impairment, 3) renal atrophy, and 4) cardiac symptoms including “unstable coronary syndrome” or “congestive heart failure”. Cardiac symptoms were defined as a New York Heart Association (NYHA) classification of II or greater. All patients underwent renal duplex ultrasonography on an outpatient basis and had ≥50% stenosis on angiography. In cases of angiographical intermediate stenosis, confirmation of a peak systolic pressure gradient ≥20 mmHg was performed using a 0.014-inch pressure wire.
Baseline clinical data were recorded as part of routine clinical practice. Systolic and diastolic blood pressure, the number of antihypertensive drugs being taken, and the levels of serum creatinine before the procedure, within 24 hours, and at follow-up (2-12 months) after renal stenting were recorded. Blood pressure was measured based on established guidelines9 and echocardiography was conducted immediately before, within 24 hours, and at follow-up after renal stenting. Changes in cardiac symptoms were also evaluated at follow-up after renal stenting.
Echocardiography measurements
Echocardiography was performed by an experienced sonographer using an Aplio SSA-770A system (Toshiba Medical Systems, Tokyo, Japan) and assessed by staff cardiologists with advanced training in echocardiography. These physicians were blinded to the other results of the study. Left ventricular ejection fraction (LVEF) was calculated using a modified Simpson’s method, and the wall motion score index (WMSI) was determined using a standard 16-segment model and a 5-point scoring system (1=normal)10. Diastolic function was measured based on peak early diastolic mitral inflow velocity (E-velocity), early filling deceleration time (DT), peak early diastolic mitral annular velocity (e’-velocity), and E/e’ ratio. The E/e’ ratio has been shown to correlate well with the LV filling pressure11-13. Mitral inflow was assessed in the apical 4-chamber view using pulsed-wave Doppler echocardiography with the Doppler beam aligned parallel to the direction of flow and the Doppler sample volume placed at the leaflet tips. The E-wave peak velocity was measured from the mitral inflow profile. Doppler tissue imaging of the mitral annulus was performed in the apical 4-chamber view using a 1- to 2-mm sample volume placed in the septal mitral valve annulus.
Interventional procedure
Primary renal artery stenting without distal protection was performed via the femoral or brachial approach under local anaesthesia. Use of a 5- to 6-mm × 15- to 18-mm Genesis or Palmaz stent (Cordis Corporation, Miami, FL, USA) was attempted. Technical success was defined as post-stent luminal narrowing of <30%. Dual antiplatelet therapy of aspirin plus clopidogrel, ticlopidine or cilostazol was administered to all patients for a minimum of two days before the procedure, and a bolus of 3500-5000 IU heparin was administered through the sheath. All patients were judged to be sufficiently hydrated to reduce the risk of contrast-media associated nephrotoxicity.
Statistical analysis
Data are expressed as a mean±SD or a number and percentage. Differences between two independent cohorts were evaluated with a Student t-test or Welch t-test for parametric continuous variables or a Mann-Whitney test for non-parametric continuous variables. Distributions of continuous variables were determined using a Shapiro-Wilk test. Categorical data were compared with a chi-square test. Multivariate logistic regression analysis was used to identify variables related to cardiac symptoms after screening for multicollinearity using Pearson correlation analysis. One-way repeated analysis of variance (ANOVA) followed by Bonferroni post hoc test was used to evaluate changes in clinical and echocardiographic variables after renal stenting. The Shapiro-Wilk test showed that the numbers of prescribed antihypertensive agents were not distributed normally. Hence, we evaluated the difference between baseline and follow-up for these data using a Wilcoxon signed rank test. Two-way repeated ANOVA was used to evaluate differences in changes of clinical and echocardiographic variables between patients with and without cardiac symptoms. The group comparison at each point was done with analysis of one-way ANOVA or Kruskal-Wallis test. A Wilcoxon signed rank test was used to compare cardiac symptoms (NYHA classification) before and after renal stenting. A probability value of P<0.05 was considered significant. SPSS version 11.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
The baseline clinical characteristics of the subjects are shown in Table 1.
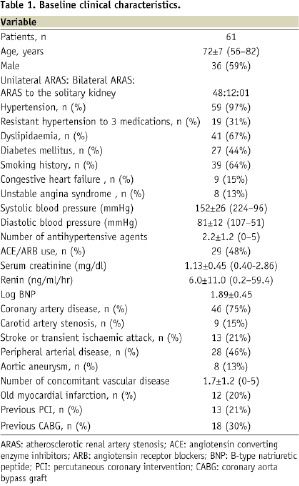
The 61 subjects underwent 73 renal stenting procedures, all of which were successful (transfemoral approach, 72; trans-brachial approach, one). Forty-eight patients suffered from unilateral ARAS, 12 from bilateral ARAS, and one from ARAS with a solitary functioning kidney due to a previous nephrectomy. Except for a chronic total occlusion in the left renal artery in one patient, all the subjects had stenosis of the ostial or proximal segment of the renal artery. There were no technical complications, major in-hospital cardiovascular events, or deaths.
Baseline echocardiography data are shown in Table 2.
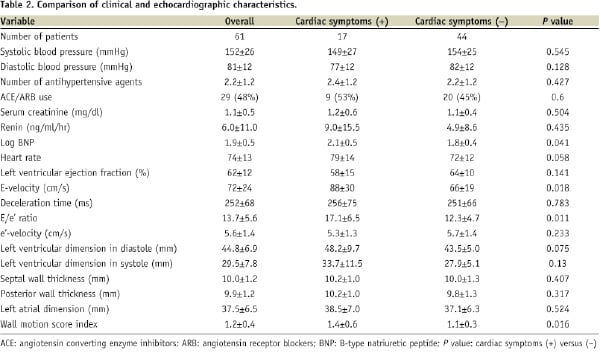
The mean LVEF was 62±12% (range, 26-81%) and the E/e’ ratio was 13.7±5.6 (range, 5.1-27.6%). The LVEF was < 50% in seven patients (11%) and the E/e’ ratio was > 15 in 20 patients (33%). Regional asynergy was observed in 20 patients (33%). Moderate or severe mitral valve regurgitation was observed in three patients (5%) and moderate aortic valve disease in two patients (3%).
Patients with cardiac symptoms had a significantly higher E/e’ ratio (P=0.011), wall motion index (P=0.016), E-velocity (P=0.018), and log B-type natriuretic peptide (BNP) level (P=0.041) compared to those without cardiac symptoms (Table 2). We found the strongest relationship (Pearson coefficient 0.854, P<0.001) between the E/e’ ratio and E-velocity. Considering the correlation between E/e’ ratio and LV filling pressure11-13, E-velocity was excluded from the multivariate regression model to avoid multicollinearity. Multivariate analysis showed that the E/e’ ratio (hazard ratio; 1.168; 95% CI, 1.007 to 1.354; P=0.040) was independently associated with cardiac symptoms (Table 3).

Effects of renal stenting
The changes in echocardiographic parameters after renal stenting are shown in Table 4.
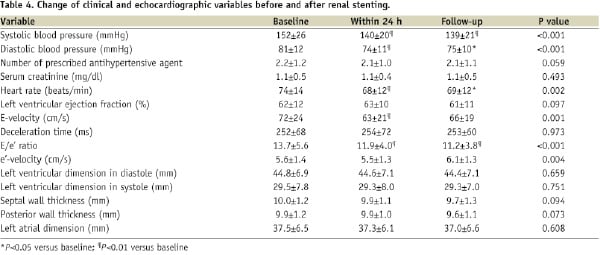
The E/e’ ratio was significantly improved after the procedure (P<0.001), whereas LVEF was unchanged. Within 24 hours after renal stenting, the E/e’ ratio decreased significantly from 13.7±5.6 to 11.9±4.0 (P=0.002). The reduction in the E/e’ ratio induced by renal stenting was sustained after a mean follow-up period of 7±4 months (range, 2–12 months), with an E/e’ ratio at this time of 11.2±3.8 (P=0.001 vs. baseline). A significant improvement in e’-velocity (P=0.004) was also observed after renal stenting, as well as a trend toward reduced septal and posterior wall thickness. These changes were accompanied by significant improvements in blood pressure, heart rate and E-velocity. Patients with cardiac symptoms showed significantly better change in E/e’ ratio (P=0.002) and E-velocity (P=0.005) after renal stenting compared to those without cardiac symptoms although there was no significant interaction between cardiac symptoms status and time (P for interaction for E/e’ ratio=0.062, P for interaction for E-velocity=0.077) (Figure 1).
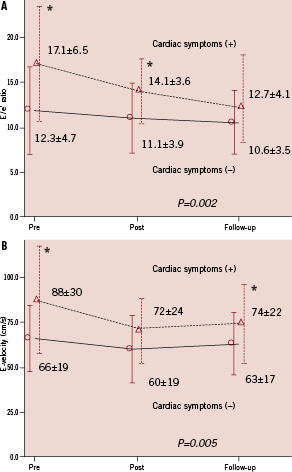
Figure 1. Changes in E/e’ ratio and E-velocity after renal stenting in patients with and without cardiac symptoms. A: E/e’ ratio, B: E-velocity. Two-way repeated ANOVA showed a significant difference in changes of E/e’ ratio (P=0.002) and E-velocity (P=0.005) between patients with and without cardiac symptoms. Error bars show standard deviations. *P<0.05: cardiac symptoms (+) versus (–) at each point.
NYHA functional class also significantly (P<0.001) improved from 2.5±0.6 at baseline to 1.4±0.6 at follow-up. However, changes of other variables did not differ significantly between patients with and without cardiac symptoms.
Discussion
The present study focused on cardiac function evaluated by echocardiography in ARAS patients before and after renal stenting. The main findings were 1) the E/e’ ratio was independently associated with the presence of cardiac symptoms in ARAS patients, 2) renal stenting contributed to a significant improvement in the E/e’ ratio and e’-velocity, and 3) there were significant differences in the responses of the E/e’ ratio and E-velocity after renal stenting between ARAS patients with and without cardiac symptoms.
Poorly controlled hypertension has been thought to initiate and promote cardiac symptoms in ARAS patients. However, Gray et al14 found no evidence of poor blood pressure control in one-third of patients with cardiac symptoms with varying levels of renal function. In the present study, the majority of ARAS patients showed preservation of LVEF, but patients with cardiac symptoms had a significantly higher E/e’ ratio, wall motion score index, E-velocity and log BNP. Furthermore, multivariate analysis identified the E/e’ ratio as the only independent predictor of cardiac symptoms. Considering that the E/e’ ratio is correlated with LV filling pressure, these findings suggest that ARAS patients with cardiac symptoms are exposed to elevated LV filling pressure, regardless of blood pressure.
Sutter et al15 first reported that a high dose of diuretics reduced the cardiac load immediately after renal revascularisation over two decades ago. Subsequent case reports and small case series have shown stabilisation of cardiac events after renal revascularisation16-18, but with a paucity of data for cardiac function. Based on these clinical observations, improvement of volume overload and diastolic dysfunction are speculated to be the main reasons for a cardiac benefit of renal revascularisation.
According to Zeller et al19, renal stenting produces regression of LV mass in patients with LV hypertrophy, in whom diastolic dysfunction is common20,21. The present study also showed a trend toward a reduction of septal and posterior wall thickness after renal stenting. Furthermore, the E/e’ ratio (which is less load-dependent) improved significantly from 13.7±5.6 to 11.9±4.0 after stenting, and remained significantly lower at 11.2±3.8 at follow-up, suggesting that preload reduction might be an integral part of the beneficial effect of renal stenting on cardiac function in both the acute and chronic stages, in addition to LV mass regression. These changes were accompanied by improvements in the load-dependent E-velocity and blood pressure, and reflect a change from moderate to mild impairment of LV filling22. These beneficial effects on cardiac function also support a previous neurohormonal study showing that BNP, a biomarker released from the ventricular myocardium under conditions causing myocardial cell stretching due to cardiac overload, is reduced after renal stenting23.
In the present study, the e’-velocity also showed significant improvement after renal stenting. ARAS can cause cardiac disorders including LV hypertrophy, volume overload and diastolic dysfunction with a broad pathophysiological spectrum from the renin-dependent phase to the volume-dependent phase24-31. A few clinical trials have found that inhibition of the renin-angiotensin-aldosterone system (RAAS) by antihypertensive drugs has a favourable impact on LV mass, myocardial fibrosis and diastolic dysfunction32-34. Considering that the e’-velocity has been reported to be a relatively load-independent parameter of LV relaxation though there is no consensus to be reached35-38, regulation of activation of the RAAS or other neurohormonal disorders by catheter-based renal revascularisation might gradually contribute to LV relaxation.
We found a significant difference in “echocardiographic” benefits of renal stenting between patients with and without cardiac symptoms. A few previous studies have reported “clinical” benefits of renal stenting on angina and heart failure. Khosla et al39 found that successful renal artery stenting improved cardiac symptoms 24 hours postprocedure in 88% of 48 patients, and there was a sustained improvement in 73% of the patients after a mean follow-up period of more than six months. Gray et al14 reported that the number of hospitalisations due to congestive heart failure was significantly reduced from 2.4±1.4 in the year preceding renal artery stenting to 0.3±0.7 in the year post-stenting (P<0.001). Thirty of 39 patients (77%) were not hospitalised for congestive heart failure during a mean follow-up period of 21 months. In the present study, the E/e’ ratio and E-velocity showed significantly more favourable responses after renal stenting in ARAS patients with cardiac symptoms compared to those without cardiac symptoms. Considering the significant improvement of cardiac symptoms with changes of these echocardiographic parameters, the cardiac benefits of renal stenting in patients with cardiac symptoms may be characterised by reduction of elevated LV filling pressure and intravascular volume that occur directly through renal artery opening.
There are several limitations that should be considered when interpreting the results of this study. First, the study was observational and involved a small number of patients without severe renal impairment at baseline. In addition, we performed TDI only on the septal side. The average e’ from multiple sites enhances the reliability of the E/e’ ratio in predicting LV filling pressures40, but almost 70% of the patients did not have regional asynergy.
In conclusion, renal stenting improved echocardiographic parameters that reflect LV diastolic function and yielded a higher benefit for E/e’ ratio and E-velocity in patients with cardiac symptoms than in those without cardiac symptoms. These results are preliminary and confirmation of the findings in a large-scale prospective study is required.
Acknowledgements
We are very grateful to Takashi Oki, MD at National Higashi Tokushima Hospital, Japan for his echocardiographic suggestions and Yasuhiro Honda, MD, Daisaku Nakatani, MD, Hiromasa Otake, MD, Teruyoshi Kume, MD and Kenji Sakata, MD at Stanford University Medical Center, USA for their statistical advice.

