Abstract
Aims: We tested the prognostic value of cystatin C in patients with documented coronary artery disease (CAD) who underwent percutaneous coronary artery intervention (PCI). We also tested the hypothesis that the incremental predictive value of cystatin C on all-cause mortality was superior to that of glomerular filtration rate (GFR) by the Modification of Diet in Renal Disease (MDRD) formula.
Methods and results: Included in the study were 2,757 patients (mean age 63 years, 77% men). Blood samples for cystatin C levels were collected immediately before PCI. During a median follow-up of two years, 114 patients died. In multivariable Cox analyses, after adjustment for several confounders, GFR (p=0.004) and cystatin C concentration (p<0.0001) were independent predictors of all-cause death. Cystatin C predicted all-cause death (c-statistic: 0.794) better than GFR estimate based on creatinine (c-statistic: 0.776, p=0.008 for comparison), and significantly reclassified 15% of patients into categories that reflected their actual likelihood of death more accurately (p=0.005). Adding cystatin C and GFR in the same multivariable survival model, only cystatin C level was a significant predictor of death.
Conclusions: This study presents for the first time the incremental predictive value of cystatin C over the creatinine-based MDRD formula on all-cause mortality for CAD patients undergoing PCI.
Introduction
Renal dysfunction has been associated with an increased risk of death and other cardiovascular complications in different clinical settings1,2. Since serum creatinine concentration is a poorly sensitive measure for detection of mild renal dysfunction3, several equations have been developed to improve the precision of assessment, but their performance is not ideal4.
Cystatin C, a 13-KDa 122-aminoacid cysteine proteinase inhibitor produced by all nucleated cells, possesses properties that make it a sensitive marker of renal function. Cystatin C is produced by cells at a constant rate, is totally filtered at glomerular level and completely reabsorbed and catabolised by the proximal tubules without any reabsorption into the blood or tubular secretion5. Cystatin C concentration is less influenced by age, gender and muscle mass than serum creatinine concentration, and therefore it may be useful for a more precise estimate of glomerular filtration rate (GFR)6,7.
In recent analyses of the Cardiovascular Health Study8-11, even mild elevations in cystatin C serum concentration predicted not only cardiovascular morbidity, but also cardiovascular, non-cardiovascular and all-cause mortality. In addition, the predictive reliability of cystatin C was independent of serum creatinine and GFR as assessed by the abbreviated Modification of Diet in Renal Disease (MDRD) formula12.
Only a few studies have analysed the potential associations between serum cystatin C and cardiovascular outcomes in patients with documented coronary artery disease (CAD)13,14. In particular, the prognostic value of serum cystatin C in patients undergoing percutaneous coronary interventions (PCI) has never been investigated. Therefore, in the present study we tested the hypothesis that the incremental predictive value of cystatin C on all-cause mortality was superior to that of the creatinine-based MDRD formula in these patients.
Methods
Our investigation was designed as a satellite analysis of the CK-MB and PCI study15, a prospective, multicentre cohort study that enrolled consecutive patients with non-ST-elevation acute coronary syndrome (ACS) or stable angina undergoing PCI at 16 Italian hospitals between February and October 2000. Briefly, the CK-MB and PCI study evaluated the influence of post-procedural CK-MB elevations on two-year all-cause mortality in a consecutive population of 3,494 patients undergoing PCI. Patients with ST-segment elevation myocardial infarction and those who refused to provide written informed consent to participate were excluded from the study. The study was approved by the local ethics committees.
Blood samples were drawn in each patient immediately before PCI (baseline). Serum was stored at –70°C and later shipped to the core biochemistry laboratory, where the biochemical markers were measured.
The patients’ vital conditions were assessed at six, 12 and 24 months after hospital discharge by means of hospital visits or phone interviews.
The central laboratory used the serum aliquots for biochemical analyses on the basis of hierarchical priorities within the CK-MB and PCI project, with the CK-MB and troponin I determinations: first, serum creatinine; second, cystatin C; and then other substudies depending on the aliquot availability16. This analysis focused on the prognostic value of pre-procedural serum levels of cystatin C. The analysis is therefore limited to 2,757 patients for whom both the creatinine and cystatin C determinations were available.
Cystatin C was measured by means of a particle-enhanced immunonephelometric assay (N Latex Cystatin C Assay; Siemens Healthcare Diagnostics, Erlangen, Germany) automatised on BN™ II nephelometer (Siemens Healthcare Diagnostics). Serum creatinine was analysed using the kinetic colourimetric Jaffé method. The upper normal limits are 1.49 mg/dL (115 mmol/L) and 1.09 mg/dL (97 mmol/L) for men and women, respectively.
We used the MDRD formula17 to estimate GFR. According to chronic kidney disease stadiation, GFR values (ml/min/1.73 m2) ≥90 were coded as stage 1 (normal), values between 60 and 89 as stage 2 (mild reduction), between 30 and 59 as stage 3 (moderate reduction), between 15 and 29 as stage 4 (severe reduction) and <15 as stage 5 (kidney failure or dialysis)18. Diabetes was diagnosed using the American Diabetes Association criteria of a fasting plasma glucose level of 7.0 mmol/L (126 mg/dL) or higher or current antidiabetic therapy. Left ventricular ejection fraction (LVEF) was determined at angiography or echocardiography. Peripheral arterial disease was defined by claudicatio intermittens associated with evidence of at least 50% peripheral artery stenosis by duplex scanning or angiography.
Follow-up
Hospital records and other source documents of patients who died were reviewed in conference by the authors of this study. All-cause mortality was considered the terminating endpoint.
Data analysis
Analyses were performed using Stata, version 12 (StataCorp LP, College Station, TX, USA) and R version 2.9.2 (R Foundation for Statistical Computing, Vienna, Austria). We expressed the continuous variables as mean value (± standard deviation [SD]) and the categorical variables as proportions. We evaluated the effect of prognostic factors on survival by univariable and multivariable Cox semiparametric regression models19,20. In univariable analyses, we tested, in addition to cystatin C concentration, the impact of several other variables known to influence mortality in this population. They included: age (years); history of diabetes (yes/no); history of hypertension (yes/no); history of dyslipidaemia (yes/no), statin prescribed at discharge (yes/no), current cigarette smoking (yes/no), prior stroke or peripheral occlusive disease (yes/no); previous coronary artery bypass graft surgery (yes/no); coronary multivessel disease (yes/no); left ventricular ejection fraction (%); admission to hospital for ACS (yes/no); unsuccessful coronary procedure (yes/no); and GFR estimated by the MDRD formula17. Multivessel disease was defined by a >50% stenosis in two or more vessels, and unsuccessful procedure was defined by a residual coronary lumen narrowing of more than 50 percent or a Thrombolysis In Myocardial Infarction (TIMI) grade flow of less than 3 after the procedure. History of hypertension was defined by the use of antihypertensive drugs or blood pressure values greater than or equal to 140 mmHg systolic or 90 mmHg diastolic. History of dyslipidaemia was defined by use of lipid-lowering drugs or total serum cholesterol >200 mg/dL.
The prognostic impact of cystatin C was also explored by conducting the analysis in the following sets of pre-planned subgroups: (i) age of patients (<65 years/≥65 years); (ii) presence of diabetes (no/yes); unsuccessful procedure (no/yes), ejection fraction (<55%/≥55%); multivessel disease (no/yes); statin prescribed at discharge (no/yes).
Subsequently, we modelled a baseline multivariable model that included some of the covariates which yielded significance in the univariable analyses. To include predictors in the baseline multivariate model, we used Akaike’s information criterion (AIC) and the Bayesian information criterion (BIC) to compare different multivariable models based on their fit to the data19.
To estimate improvement in the predictive ability by reducing the unexplained variance, separately to the baseline model, we added GFR estimate by MDRD equation and serum cystatin C concentration. We tested the incremental value of GFR estimated by the MDRD equation and cystatin C, calculating Harrell’s c-statistic21 and the net reclassification improvement (NRI) according to Pencina22,23. We also calculated the receiver-operating characteristic (ROC) curves and compared cystatin C to GFR assessed by MDRD (differences between the areas under the curves, AUC). Two-sided p-values ≤0.05 were considered statistically significant.
Results
Overall, 2,757 patients were included in the present analysis. They were admitted to hospital for ACS (non-ST-elevation ACS, 51.3%) or for stable angina (48.7%) with indications for coronary angiography and PCI with stent placement. Unsuccessful percutaneous coronary intervention was recorded in 132 (4.8%) patients.
Follow-up data, including the survival status, were available for all patients (100%).
The main characteristics of the population at entry are shown in Table 1. Mean age was 63.3 years. Prevalence of diabetes, hypertension and dyslipidaemia were 19.7%, 58.9% and 58.6%, respectively.
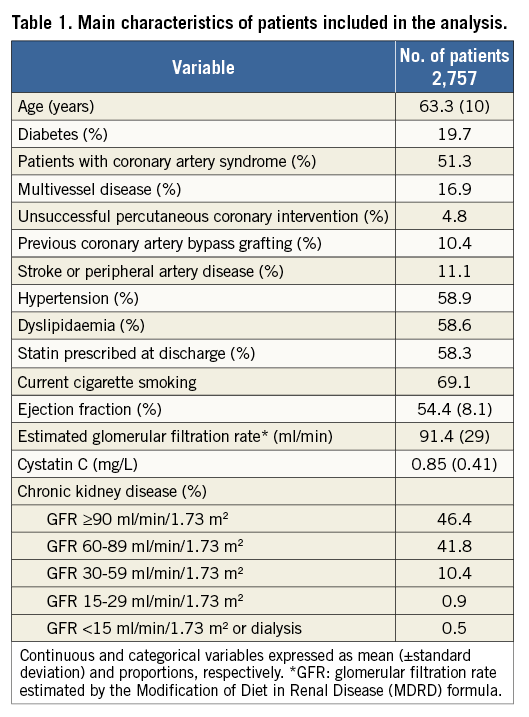
GFR was normal (stage 1) in 46.4%, mildly reduced (stage 2) in 41.8%, moderately reduced (stage 3) in 10.4% and severely reduced (stage 4) in 0.9%, while 0.5% of patients had end-stage kidney failure or dialysis.
During a median follow-up period of two years (interquartile range: 1.99-2.07), 114 patients (4.1%) died. The ROC curve analysis for cystatin C levels for all-cause mortality is reported in Figure 1. Cystatin C concentration showed a good discriminating power between patients with and without subsequent death: the area under the ROC curve was 0.708 (95% confidence interval [CI]: 0.653-0.763; p=0.033) for cystatin C and 0.623 (95% CI: 0.563-0.683; p=0.020) for GFR estimated by the MDRD formula (difference between areas: 0.09, p=0.005).
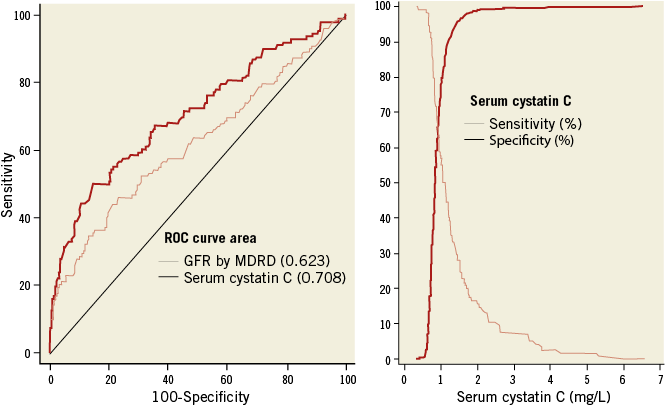
Figure 1. Areas under the curve with cystatin C and glomerular filtration rate estimated by MDRD formula to test their different ability in the prediction of all-cause mortality (left panel). The crossing point (right panel) between sensitivity and specificity curves for total mortality with cystatin C was 1.02 mg/L.
Table 2 shows the results of univariable Cox analyses. The risk of death decreased with higher GFR estimated by MDRD formula with a hazard ratio and 95% CI for each increment of 10 ml/min/1.73 m2 equal to 0.81 (0.75-0.87, p<0.0001). For each 0.1 mg/L increase in cystatin C levels there was a 9% higher risk of death (95% CI: 7-10; p<0.0001). Among other predictors of all-cause death which yielded significance in the univariable analyses, age (years), diabetes (yes/no), unsuccessful coronary procedure (yes/no), ejection fraction (%), multivessel disease (yes/no) and statin prescribed at discharge (yes/no) were included in a baseline multivariable model (Table 3). Other tested covariates did not achieve the significance to enter the final baseline model (all p>0.05).
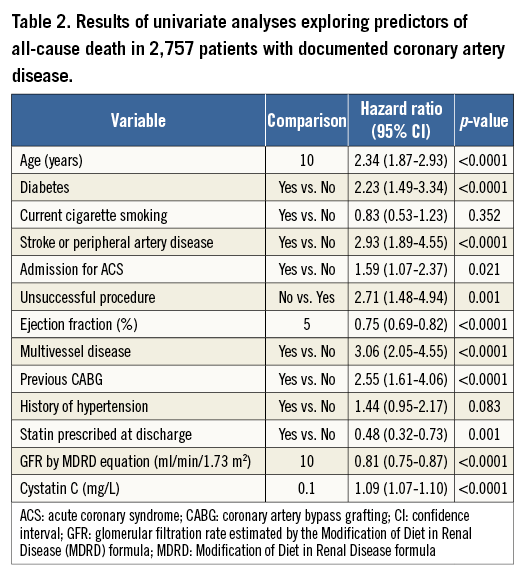
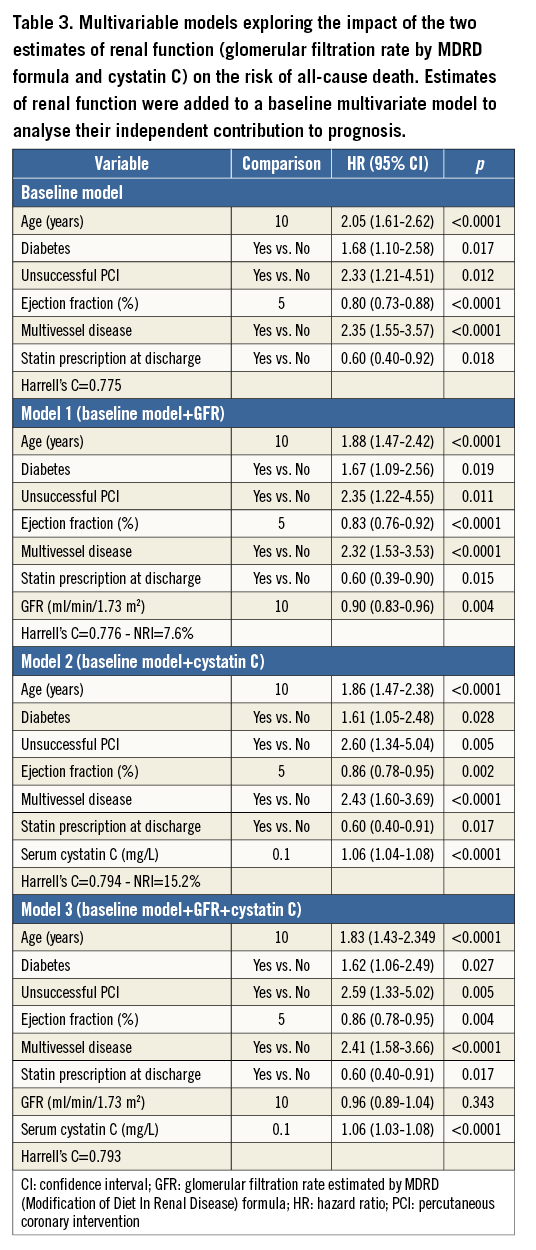
When added separately to the baseline model, GFR estimated by MDRD equation and serum cystatin C concentration improved the predictive ability by reducing the unexplained variance (likelihood ratio tests: p<0.001 for each). Risk of death decreased with higher GFR estimated by MDRD formula: the multivariable hazard ratio (HR) and 95% confidence intervals for each increment of 10 ml/min/1.73 m2 in GFR was 0.90 (0.83-0.96, p=0.004). Similarly, serum cystatin C concentration was an independent predictor of all-cause mortality (p<0.0001) after adjustment for the significant effect of: age (p<0.0001), diabetes (p=0.028), ejection fraction (p=0.002), multivessel disease (p<0.0001), unsuccessful procedure (p=0.005) and statin prescribed at discharge (p=0.017). For each increment of 0.1 mg/L in serum level of cystatin C there was a 6% significant increased risk of death (95% CI: 4-8%, p<0.0001).
The c-statistics increased from 0.775 (baseline model) to 0.776 with the GFR estimated by MDRD equation, and 0.794 with cystatin C levels. Renal function estimated by cystatin C performed better than that of the MDRD equation (p=0.008 for comparison).
To evaluate the incremental predictive value of cystatin C further, we forced cystatin C and GFR estimated by MDRD equation in the same model. When added simultaneously to the model, only cystatin C was a significant predictor of all-cause mortality (Table 3). For each increment of 0.1 mg/L in serum level of cystatin C there was a 6% significant increased risk of death (95% CI: 3-8%, p<0.0001).
Stratified analyses proved that the impact of cystatin C on the risk of death was consistent in all the pre-planned subgroups defined by age, presence of diabetes, outcome of coronary intervention, ejection fraction, presence of multivessel disease, and statin prescribed at discharge (Figure 2). In addition, no significant interactions were observed between cystatin C concentration and covariates included in the baseline multivariable model (all p>0.05).
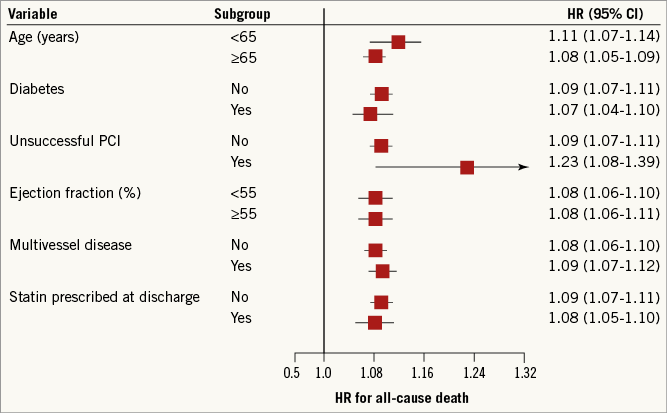
Figure 2. Pre-planned subgroup analyses evaluating the prognostic impact on all-cause death of cystatin C. Hazard ratios and 95% confidence intervals were computed for each increment of 0.1 mg/L in serum level of cystatin C. CI: confidence interval; PCI: percutaneous coronary intervention
We then calculated the net reclassification improvement according to Pencina22,23, to investigate whether GFR estimated by MDRD formula and cystatin C level reclassified patients into categories that reflected their actual likelihood of death better. We identified four categories of death (<5%; 5-10%; 10-20%; >20%) according to the GISSI-Prevenzione risk score24. The proportion of patients correctly reclassified into lower or higher risk categories was 7.6% (p=0.149) with the GFR estimated by MDRD formula, and 15.2% (p=0.005) with cystatin C levels. For cystatin C, the detailed proportions of reclassified patients are shown in the Figure 3.
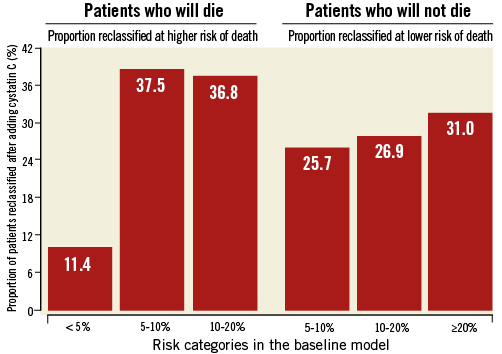
Figure 3. Reclassification of patients into categories at higher or lower risk of death by cystatin C levels. Overall, the proportion of patients correctly reclassified into lower or higher risk categories was 15.2%.
Discussion
Our study extends to CAD patients undergoing PCI the bulk of prior evidence on the prognostic impact of cystatin C in different clinical settings8-10,12-14,25. Our data also suggest that serum cystatin C measurements at the time of PCI refine prognostic stratification in these patients. Notably, we observed that the association between cystatin C concentration and mortality remained significant after adjustment for several covariates including age, diabetes, unsuccessful coronary procedure, ejection fraction, statin treatment and extension of CAD.
In addition, our results suggest that the incremental predictive value for all-cause mortality of cystatin C concentration might be superior to that of the creatinine-based MDRD formula. Cystatin C levels predict all-cause death better than estimates based on creatinine and significantly reclassified 15% of patients into categories that reflected their actual likelihood of death better.
Previous studies
To our knowledge, only three studies have examined the prognostic impact of cystatin C in patients with established CAD. None of these studies was conducted in patients undergoing PCI. Koenig and co-workers, following a cohort of 1,033 CAD patients for an average of 33.5 months, showed that patients in the top quintile of cystatin C concentration showed an increased risk of the composite outcome of fatal and nonfatal cardiovascular events (HR 2.27; 95% CI: 1.05-4.91), even after adjustment for traditional risk factors and the severity of CAD14. Similarly, Jernberg and co-workers13 observed that ACS patients in the third and fourth quartile of the distribution of cystatin C concentration had a significantly increased risk of death (HR 3.2, 95% CI: 1.2 to 8.5, and HR 11.7, 95% CI: 4.7 to 29.3, respectively) when compared with patients in the bottom quartile.
Ix and co-workers25 examined a mixed population of 990 patients with a history of either myocardial infarction, angiographic evidence of coronary stenosis, exercise-induced ischaemia or prior coronary revascularisation. After a mean follow-up period of 37 months, 132 patients died, 101 had cardiovascular events and 57 had incident heart failure. Serum cystatin levels in the upper quartile were associated with a significant excess risk of all the above end-points independently of estimated GFR and microalbuminuria25.
Potential mechanisms
The mechanisms through which elevated cystatin C levels relate to long-term mortality remain elusive. Serum cystatin C concentration reflects the balance of its primary physiological determinants: cellular generation, renal filtration, and subsequent renal degradation without any systemic reabsorption26. Serum cystatin levels increase with age, being higher in males than in females and in non-Hispanic persons27. The generation of cystatin C by nucleated cells is constant and it is not influenced by age, sex, height, and body composition6,26. Therefore, it is believed that an increased cystatin C concentration may identify subjects with early impaired renal function and increased associated risk of cardiovascular disease2,8,9. However, agreement is not univocal. Knight and co-workers examined a large cohort of 8,058 individuals and found that older age, male gender, greater weight, greater height, current cigarette smoking, and higher serum C-reactive protein levels were independently associated with higher serum cystatin C levels, even after adjusting for creatinine clearance28.
Currently, it is not quite clear whether cystatin C is a prognostic marker solely because it is a more sensitive indicator of renal function, or because it also reflects adverse pathogenetic mechanisms that are partially or totally independent of renal function. For example, there is evidence that inflammatory status, thyroid disease, serum C reactive protein, and current smoking were all associated with cystatin C concentrations29,30. Inflammatory cytokines may alter the balance between lysosomal cathepsins and their endogenous inhibitors, like cystatin C, thus favouring atherogenesis29. Elastolytic cysteine proteases and their inhibitors, including cystatin C, are believed to be involved in the pathogenesis of atherosclerosis31. This may explain in part the mechanism underlying the statistical link between cystatin C and cardiovascular outcomes in subjects with normal creatinine-based GFR. Thus, increases in serum cystatin C may be sensitive harbingers of cardiovascular disease that are associated with inflammation and atherosclerosis32. As with creatinine estimation, cystatin C measurement by means of the recent standardisation process33 may be routinely carried out on several general clinical chemistry analysers, allowing the wide diffusion and easier clinical utilisation of the test. Although more expensive than serum creatinine, measurement of cystatin C could be adopted not only as a diagnostic test for kidney function, but, more importantly, as a prognostic mortality tool among patients with CAD undergoing percutaneous coronary intervention.
Limitations of the study
Since this study was conducted in a Caucasian population, results may not be extrapolated to different ethnic groups. We assessed all-cause mortality (we were unable to collect enough information on softer endpoints) during a follow-up of two years, and extrapolation of results to longer periods requires caution. Furthermore, although all patients underwent PCI with stent placement and unsuccessful percutaneous coronary intervention was recorded in 132 patients, other related features of PCI were not originally collected in our database.
Finally, we could not compare the prognostic value of cystatin C with that of microalbuminuria, which also reflects an early impairment of renal function34.
Conclusions
The current study extends for the first time to the growing population of CAD patients undergoing PCI the evidence that a single cystatin C evaluation before the procedure is a strong and independent predictor of mortality over a two-year period. In addition, the performance of cystatin C as a prognostic marker might be superior to that of the creatinine-based MDRD formula.
| Impact on daily practice The estimated glomerular filtration rate (GFR) is the clinical standard for the assessment of kidney function. Nevertheless, cystatin C has received much attention as an alternative filtration marker with stronger and more linear risk relationships than creatinine or GFR. Our analysis demonstrates the prognostic impact of cystatin C in patients with coronary artery disease (CAD) treated with percutaneous coronary intervention and suggests its ability to refine prognostic stratification above and beyond clinical risk factors and the traditional GFR estimation. From a practical standpoint, it may be worth adding cystatin C as a second measure of kidney function to identify CAD patients at increased cardiovascular risk needing an aggressive secondary prevention strategy and an optimal long-term management. |
Acknowledgements
This study has been supported in part by an unrestricted grant from Bracco Imaging S.p.A., Milan. The study has also been supported by the Fondazione Umbra Cuore e Ipertensione – ONLUS, Perugia, Italy.
Conflict of interest statement
The authors have no conflicts of interest to declare.

