Abstract
Aims: Bivalirudin has emerged as a meaningful alternative to heparin in patients undergoing percutaneous coronary intervention (PCI). To date, it is unclear whether bivalirudin has advantages in patients undergoing rotational atherectomy (RA).
Methods and results: The current subgroup analysis of the ROTAXUS trial compared patients receiving bivalirudin (n=129) to those receiving unfractionated heparin (UFH) (n=111). Efficacy was assessed by the frequency of periprocedural myocardial infarction (MI) and safety by the frequency of major access-site bleeding (ASB). Baseline characteristics were similar. Periprocedural MI occurred less frequently in the bivalirudin group (22% vs. 37.5%, p=0.02), while ASB did not differ significantly (2.3% vs. 5.5%, p=0.20). This effect was larger in the RA group, where bivalirudin significantly reduced periprocedural MI (15.7% vs. 38.7%, p=0.01) with a trend towards reduced major ASB (2.9% vs. 10.2%, p=0.09). In the control group without RA, bivalirudin was not superior to UFH regarding periprocedural MI (28.6% vs. 36.6%, p=0.42) and major ASB (1.7% vs. 1.7%, p=0.99).
Conclusions: This analysis suggests a differential benefit of bivalirudin in patients treated with RA. Patients receiving bivalirudin during RA showed significantly less periprocedural MI and fewer ASB compared to patients treated with UFH.
Abbreviations
ACT: activated clotting time
ARC: Academic Research Consortium
ASB: access-site bleeding
MACE: major adverse cardiac events
MI: myocardial infarction
PCI: percutaneous coronary intervention
RA: rotational atherectomy
TLR: target lesion revascularisation
TVR: target vessel revascularisation
UFH: unfractionated heparin
Introduction
Percutaneous coronary intervention (PCI) of heavily calcified lesions, which are predominantly seen in elderly patients with advanced coronary artery disease, have been associated with lower procedural success rates, an increased frequency of acute complications, and higher restenosis rates than PCI of non-calcified lesions1,2. In clinical practice, unfractionated heparin (UFH) has been the standard anticoagulant administered during PCI with a target activated clotting time (ACT) of 250-300 seconds3. In large randomised trials, the use of bivalirudin, a direct thrombin inhibitor, has been associated with lower rates of bleeding events and comparable efficacy compared to UFH in patients who undergo PCI for a variety of clinical syndromes4-6, but data concerning both safety and efficacy in patients with complex calcified lesions treated with or without rotational atherectomy are scarce. This pre-specified subanalysis of the prospective multicentre ROTAXUS (Rotational Atherectomy Prior to TAXUS Stent Treatment for Complex Native Coronary Artery Disease) trial7 compares the periprocedural and long-term outcome of patients undergoing complex PCI with or without rotational atherectomy (RA) treated by either UFH or bivalirudin.
Methods
PATIENTS AND STUDY DESIGN
The ROTAXUS trial design has been previously published7. In brief, between August 2006 and March 2010, 240 eligible patients with complex calcified native coronary artery disease were randomised 1:1 to a strategy of rotablation followed by stenting or stenting without prior rotablation (standard therapy). Stenting was performed using the polymer-based slow-release paclitaxel-eluting TAXUS stent (PES; Boston Scientific, Boston, MA, USA). The primary endpoint of the trial was in-stent late lumen loss at nine-month angiography.
PROCEDURE
After randomisation, patients received 325-500 mg aspirin intravenously or orally (if they did not receive it within the previous 12 hours) and an oral loading dose of 600 mg clopidogrel. Procedural antithrombotic treatment strategy, which was left to the discretion of the operator, was carried out by UFH or bivalirudin. UFH was given intra-arterially at a dose of 70-100 U/kg and was guided by ACT (250-300 seconds). Bivalirudin was used as an initial intravenous bolus of 0.75 mg/kg, followed by an intravenous infusion of 1.75 mg/kg/hr given only for the duration of the procedure. Glycoprotein IIb/IIIa inhibitors were only used for bail-out conditions. Rotational atherectomy was performed by the Rotablator (Boston Scientific Scimed Inc., Maple Grove, MN, USA). The burr size was selected to reach a burr/vessel ratio of 0.5 (max. 0.7 if needed). Rotablation speed ranged between 140,000 and 180,000 rotations per minute. A temporary pacemaker wire was inserted during rotablation of the right coronary artery and the left circumflex artery in patients with a dominant left system. All procedures were performed transfemorally and closure devices were used in all patients (Starclose SE®; Abbott Vascular, Santa Clara, CA, USA). Venous access was compressed manually and thereafter supplied with a pressure bandage for three hours.
FOLLOW-UP AND DEFINITIONS
After PCI, all patients received 100 mg of aspirin daily indefinitely and 75 mg of clopidogrel for at least six months. An electrocardiogram (ECG) was performed 24 hours post procedure or prior to discharge, whichever occurred first. In addition, troponin T, creatine kinase (CK) and CK-myocardial band (MB) were measured 8-12 hours post procedure. If any elevation was noted, additional measurements were performed as needed. During hospital stay, patients were clinically monitored for the occurrence of any adverse events and any additional coronary interventional treatment. At nine months, a clinic visit and a repeat coronary angiography were performed. A clinical events adjudication committee adjudicated all major cardiac events.
Major adverse cardiac events (MACE) were defined as the composite of death, new myocardial infarction (MI) and target vessel revascularisation (TVR). Death was defined as all-cause mortality. Protocol-defined MI was defined as elevated CK ≥3 times the upper limit of normal with elevated CK-MB (any amount above the institution’s upper limit of normal) in the absence of new pathological Q-waves (non-Q-wave MI), or as chest pain or other acute symptoms consistent with myocardial ischaemia, and/or new pathological Q-waves in two or more contiguous ECG leads in the absence of timely cardiac enzyme data, and/or new pathological Q-waves in two or more contiguous leads and elevation of cardiac enzymes (Q-wave MI). Target vessel revascularisation (TVR) was defined as a repeated procedure, either PCI or bypass surgery, on the target vessel, and target lesion revascularisation (TLR) was defined as any reintervention inside the stent implanted during the index procedure or within 5 mm proximal or distal to the stent. Stent thrombosis was defined as proposed by the Academic Research Consortium (ARC)8. Angiographic success was defined as successful stent delivery and expansion with attainment of <20% in-stent residual stenosis of the target lesion in the presence of TIMI 3 flow, whereas strategy success was defined as angiographic success without crossover or stent loss.
Efficacy and safety endpoints of the current subanalysis were the occurrence of periprocedural MI and access-site bleeding (ASB), respectively. Periprocedural MI (type 4a) was defined according to the universal definition9 with a troponin T elevation >3 times the 99th percentile upper limit of normal. ASB was defined according to the ACUITY trial10, which included ASB requiring interventional or surgical correction, haematoma ≥5 cm, retroperitoneal bleeding, or haemoglobin drop ≥3 g/dL with ecchymosis or haematoma <5 cm, oozing blood, or prolonged bleeding (>30 minutes) at the access site.
STATISTICAL METHODS
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS for Windows, Version 13; SPSS Inc., Chicago, IL, USA). Continuous data are presented as mean (SD) or median (IQR). Categorical data are presented as counts and proportions (%). Differences between groups were checked for significance with the Student’s t-test or Wilcoxon rank-sum test (continuous data) or the chi-squared or Fisher’s exact test when the expected cell value was less than 5 (categorical variables). A logistic regression analysis was performed to test for an interaction between bivalirudin therapy and the use of RA. All tests were two-sided and a p-value of <0.05 was considered statistically significant.
Results
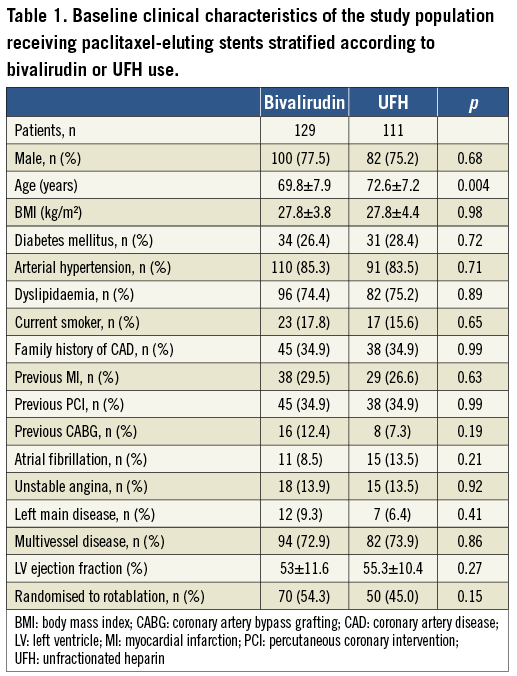
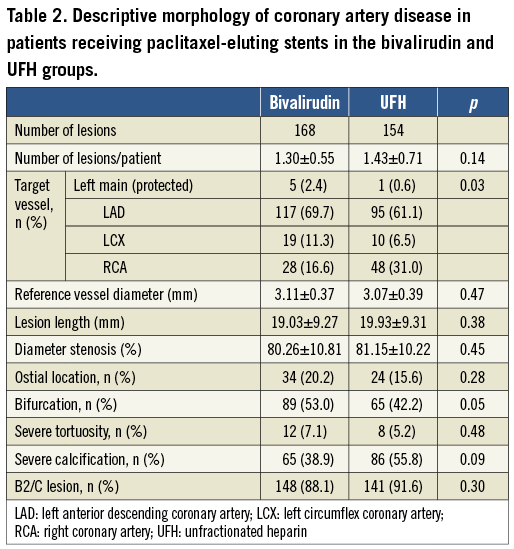
Between 2006 and 2010, a total of 240 patients were prospectively enrolled at three German sites. Of these, 129 patients were treated with bivalirudin and 111 patients were treated with UFH. Baseline clinical characteristics were comparable between the groups, but patients in the UFH group were older (72.6±7.2 years vs. 69.8±7.9 years; p=0.004) (Table 1). The comorbidities in both groups mirrored the unselected nature of such patients, with one quarter of patients being diabetics, one third with previous PCI, two thirds with multivessel disease, and an overall well-preserved left ventricular ejection fraction. Angiographic characteristics are shown in Table 2. Around 90% of patients in both groups had a type B2/C lesion morphology. Nearly two thirds of procedures were performed at the LAD with a mean lesion length of 20 mm and a mean vessel diameter of 3.1 mm.
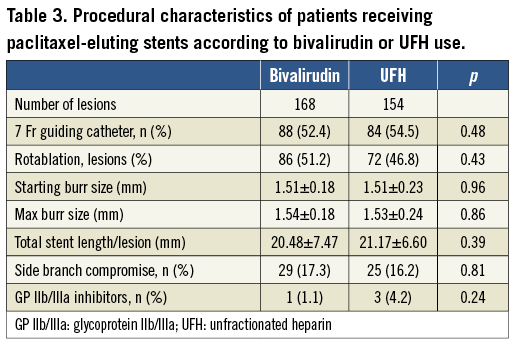
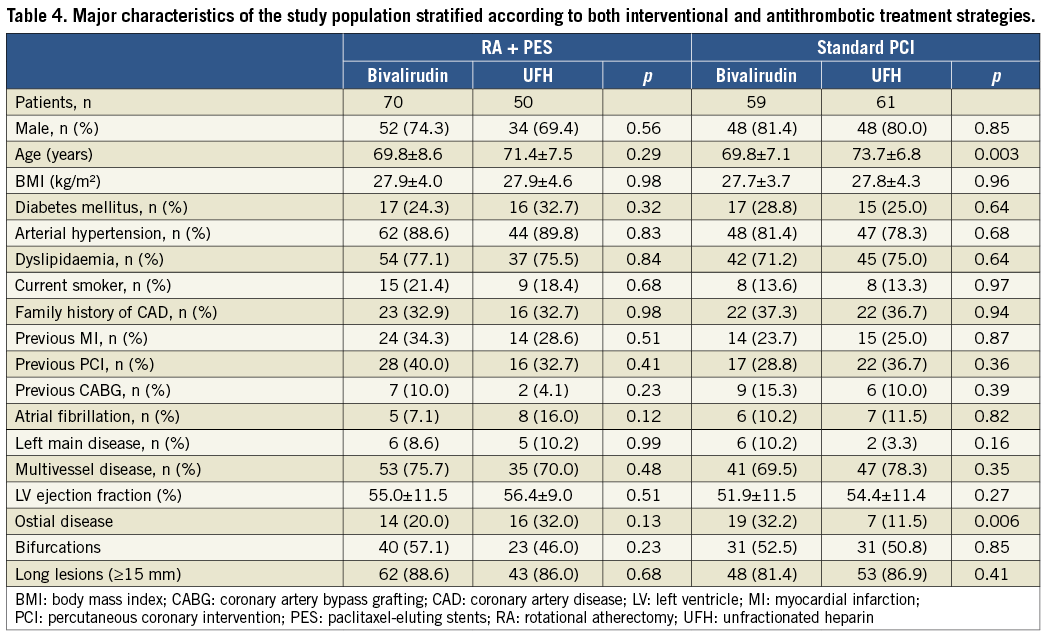
In the bivalirudin and UFH groups, respectively, 86 (51.2%) and 72 (46.8%) lesions were treated with rotablation without any significant differences in procedural characteristics. Most rotablation procedures were performed through a 7 Fr arterial sheath and an additional 6 Fr venous access for a pacing lead. Treatment strategies were similar without any differences regarding burr size and GP IIb/IIIa inhibitor use (Table 3). The overall angiographic and strategy success rates were 97% vs. 97.4% (p=0.84) and 91.1% vs. 86.4% (p=0.18) in the bivalirudin and UFH groups, respectively. Major characteristics of the study population stratified according to both interventional (RA vs. standard therapy) and antithrombotic treatment (bivalirudin vs. UFH) strategies are shown in Table 4.
In-hospital follow-up revealed similar rates for total mortality, protocol-defined MI, TVR, MACE as well as definite stent thrombosis in both the bivalirudin and the UFH groups (Table 5). Similarly, clinical follow-up at nine months revealed no differences with respect to mortality, MI, and TVR (Table 5).
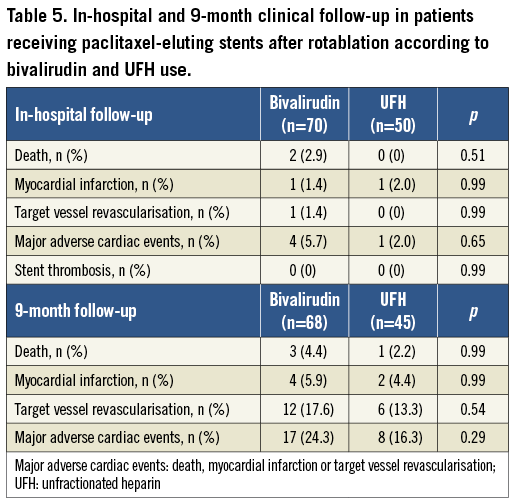
Post-procedural troponin T levels (8-12 hours post PCI) were analysed in 172 patients with normal baseline values and showed a similar incidence of any troponin T elevation (45.1% vs. 50%, p=0.52) and troponin T elevation >3 times above the upper limit of normal (24.4% vs. 32.2%, p=0.26) in the whole RA and standard therapy groups, respectively. However, a stratification according to antithrombotic treatment strategy revealed that troponin T elevation >3 times above the upper limit of normal was significantly lower in patients treated with bivalirudin compared to UFH (22.0% vs. 37.5%; p=0.02), driven by significantly lower events in the rotablation group (15.7% vs. 38.7%; p=0.01), with no differences in the standard PCI group (28.6% vs. 35.6%) (Figure 1). Using logistic regression analysis, there was a suggestion of an interaction between bivalirudin therapy and RA regarding periprocedural MI, but this was not statistically significant (interaction p-value=0.22, odds ratio=0.42, 95% confidence interval=0.11-1.67). This remained unchanged when the analysis was adjusted for various confounders including age, sex, diabetes mellitus, ostial location, bifurcation and long lesions.
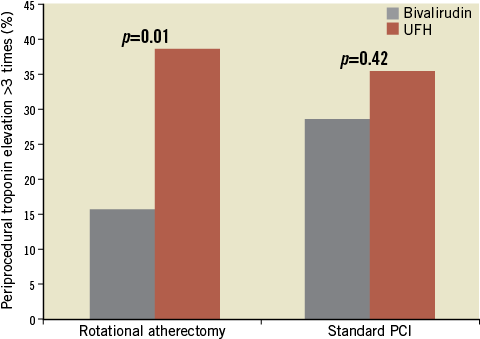
Figure 1. Periprocedural myocardial infarction (efficacy parameter) in the rotational atherectomy and standard PCI groups stratified according to the antithrombotic treatment strategy (data extracted from 172 patients with normal baseline troponin values).
As for major ASB, defined as a safety parameter, there was a trend for lower events in favour of bivalirudin in the rotablation group (2.9% vs. 10.2%; p=0.09), with equal rates in the standard PCI group (1.7% vs. 1.7%; p=0.99), resulting in non-significant values for the whole ROTAXUS study population (Figure 2).
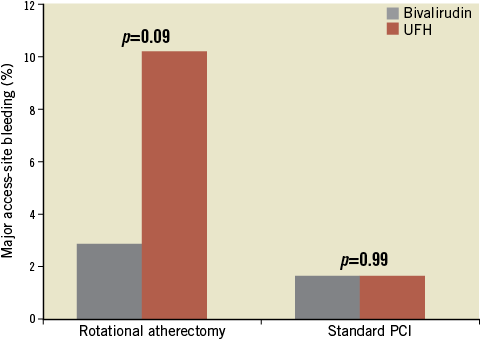
Figure 2. Major access-site bleeding (safety parameter) in the rotational atherectomy and standard PCI groups stratified according to the antithrombotic treatment strategy.
Discussion
The current analysis of the prospective multicentre ROTAXUS trial revealed significantly lower rates of periprocedural MI with bivalirudin as compared with UFH in patients undergoing complex PCI with the use of RA. Furthermore, there was a trend in favour of bivalirudin in reducing major ASB.
The present report, which is the first differential analysis about bivalirudin in patients treated with rotational atherectomy, is in line with previous trials comparing antithrombotic treatment strategies in different non-rotablation settings4-6,11,12. REPLACE-2 evaluated bivalirudin versus UFH/GPIs in patients undergoing elective PCI4 and found that bivalirudin was non-inferior to UFH/GP IIb/IIIa inhibitors with regard to the composite endpoint of death, MI, TVR, and bleeding, whereas treatment with bivalirudin resulted in significantly lower major bleeding rates than UFH/GP IIb/IIIa inhibitors (2.4% vs. 4.1%; p<0.001). The use of bivalirudin is recommended by various national and international guidelines and is a class I recommendation in patients with acute coronary syndromes undergoing PCI13,14. Several studies have demonstrated that bleeding complications are strongly associated with short- and long-term mortality after PCI15-18. In their meta-analysis, Verheugt et al compared data from the REPLACE-2, ACUITY, and HORIZONS-AMI trials in 17,393 PCI patients19. The composite of major and minor bleeding, defined according to the TIMI criteria, occurred in 5.3% of patients. After risk adjustment, TIMI bleeding was associated with an increased risk of one-year mortality (hazard ratio [HR]: 3.17, 95% confidence interval [CI]: 2.51 to 4.00, p<0.0001). ROTAXUS, which used the ACUITY definition10 for describing bleeding events, represents a head-to-head comparison between bivalirudin and UFH, excluding possible confounding factors such as different prevalence of GP IIb/IIIa inhibitors in the two arms or periprocedural UFH doses exceeding the current guidelines. The lack of significant difference in safety endpoints in our whole cohort could be explained by the low number of patients and events but also by the dosage of heparin as well as the use of access-site closure devices with subsequent pressure bandage. However, there was a trend towards lower ASB in patients receiving bivalirudin allocated to RA, which might be due to use of larger access sheaths and, in certain patients, use of additional 6 Fr venous access for pacing. However, due to the different bleeding definitions in several trials, a comparison is not reliable. For a unification of these differences, the Bleeding Academic Research Consortium (BARC) definition was recently introduced20,21.
The incidence of elevated biomarkers of myocardial damage after PCI is 1-48%17,22-25. This variation reflects differences in the biomarker measured and the patient populations. There is an increasing consensus that troponin is probably the most sensitive biomarker, but the prognostic impact of troponin elevation after PCI is still debated23,24. The universal definition of MI used in this subanalysis arbitrarily defines “PCI-related MI” as patients with normal baseline levels and a rise of troponin three times the 99th percentile of the upper reference limit (URL)9. Due to the possibility of overdiagnosis of clinically relevant events, this cut-off was recently changed to five times the 99th percentile of URL26. It remains possible that the use of the more sensitive definition may have contributed to the differences seen in ROTAXUS, especially given that the rates of the more conservative protocol-defined MI were similar.
Extensive atherosclerotic plaque burden, intraluminal thrombus, multivessel interventions, rotablation use, larger balloon/artery ratio, development of no-reflow, side branch occlusion, abrupt vessel closure, and atherothrombotic or platelet embolisation have been found to be related to postprocedural enzyme elevations25. Studies have shown that CK-MB elevation during rotational atherectomy can occur in up to 15% to 30%27. Notably, the rate of MI was not higher in the RA group of ROTAXUS. ROTAXUS, however, revealed a significantly lower rate of periprocedural MI with bivalirudin as compared to UFH, a benefit that appeared to be larger in patients treated with RA, despite the lack of a significant statistical interaction. Differences in the mechanism of RA and balloon angioplasty and its interaction with UFH and bivalirudin might explain these findings. The occurrence of thrombus formation in standard treatment is primarily due to morphological factors like side branch occlusion and dissection4-6. In patients treated with high-speed RA, the abraded plaque is pulverised into microparticles, which are 5-10 µm in diameter28. The debris created by the atherectomy devices includes atheroma plus platelet aggregates which may clot in the distal microcirculation, potentially causing haemodynamic and ischaemic complications, and are probably responsible for periprocedural troponin and CK-MB release. Accordingly, Kini et al reported on the beneficial effect of abciximab after rotational atherectomy29. Similarly, it appears from the current analysis that these atherothrombotic aggregates are more effectively prevented by bivalirudin than by UFH.
Study limitations
The present study has several limitations which ought to be emphasised. The treatment strategy was left to the discretion of the operator, which might be biased by lesion morphology and clinical setting. We have no information on the rate of non-access-site bleeding and its management. However, our aim was to address the periprocedural events in case of complex coronary intervention with the use of different treatment strategies (UFH versus bivalirudin) and not to address the long-term efficacy and safety of both treatment strategies. Due to the small number of patients who suffered from clinical events during follow-up, there is a lack of power for evaluating the impact of bleeding prevention by bivalirudin on mortality reduction. Additionally, all patients in our study underwent PCI using the femoral approach and, with the increasing application of radial access, entry-site complications may be expected to decrease, regardless of the pharmacology used.
Conclusions
This subgroup analysis of the ROTAXUS trial suggests a differential benefit of bivalirudin in patients treated with RA. Patients receiving bivalirudin during RA showed significantly less periprocedural MI and a strong trend towards fewer access-site complications compared to patients receiving UFH. However, this periprocedural benefit did not translate into a clinical benefit at nine months, which might be due to a limited relevance of these outcome measures or to the rather low number of patients and events. An adequately powered randomised controlled trial in patients undergoing PCI with rotablation stratified to both antithrombotic treatment strategies is warranted.
| Impact on daily practice Rotational atherectomy is an effective tool for preparation of heavily calcified lesions that cannot be crossed by a balloon or adequately dilated before planned stenting. Due to an interplay of different factors, coronary microcirculatory disturbance may occur with rotational atherectomy, which could result in periprocedural myocardial infarction. The current study suggests an advantage of the direct thrombin inhibitor bivalirudin compared to the standard therapy of unfractionated heparin in preventing periprocedural myocardial infarction with rotational atherectomy. Bivalirudin also showed a trend towards fewer access-site complications. These findings may influence the choice of adjunctive antithrombotic therapy in the setting of percutaneous coronary intervention of heavily calcified lesions, but need to be confirmed in a dedicated randomised trial. |
Conflict of interest statement
M. Abdel-Wahab, G. Richardt and V. Geist report supporting educational activities and receiving lecture fees from Boston Scientific. All the other authors report no conflicts of interest in relation to the contents of this manuscript.

