
Abstract
Aims: This study aimed to evaluate associations between coronary collaterals and myocardial viability as assessed by quantitative cardiac magnetic resonance (CMR) imaging in patients with a chronic coronary total occlusion (CTO).
Methods and results: A total of 218 patients with a CTO who underwent CMR between 2013 and 2018 were included. A concomitant collateral connection (CC) score 2 and Rentrop grade 3 defined well-developed collaterals in 146 (67%) patients, whereas lower CC scores or Rentrop grades characterised poorly developed collaterals. Dysfunctional myocardium (<3 mm segmental wall thickening [SWT]) and ≤50% late gadolinium enhancement (LGE) defined viability. Extensive scar (LGE >50%) was observed in only 5% of CTO segments. In the CTO territory, SWT was greater (3.72±1.51 vs 3.05±1.60 mm, p<0.01) and the extent of scar was less (7.0 [0.1-16.7] vs 13.1% [2.8-22.2], p=0.048) in patients having well-developed versus poorly developed collaterals. Viability was more prevalent in CTO segments among patients with poorly developed versus well-developed collaterals (44% vs 30% of segments, p<0.01), predominantly due to a higher prevalence of dysfunctional myocardium (51% vs 34% of segments, p<0.01) in the poorly developed collateral group.
Conclusions: The infarcted area in myocardium subtended by a CTO is generally limited. Well-developed collaterals are associated with less myocardial scar and enhanced preserved function. However, viability was regularly present in patients with poorly developed collaterals.
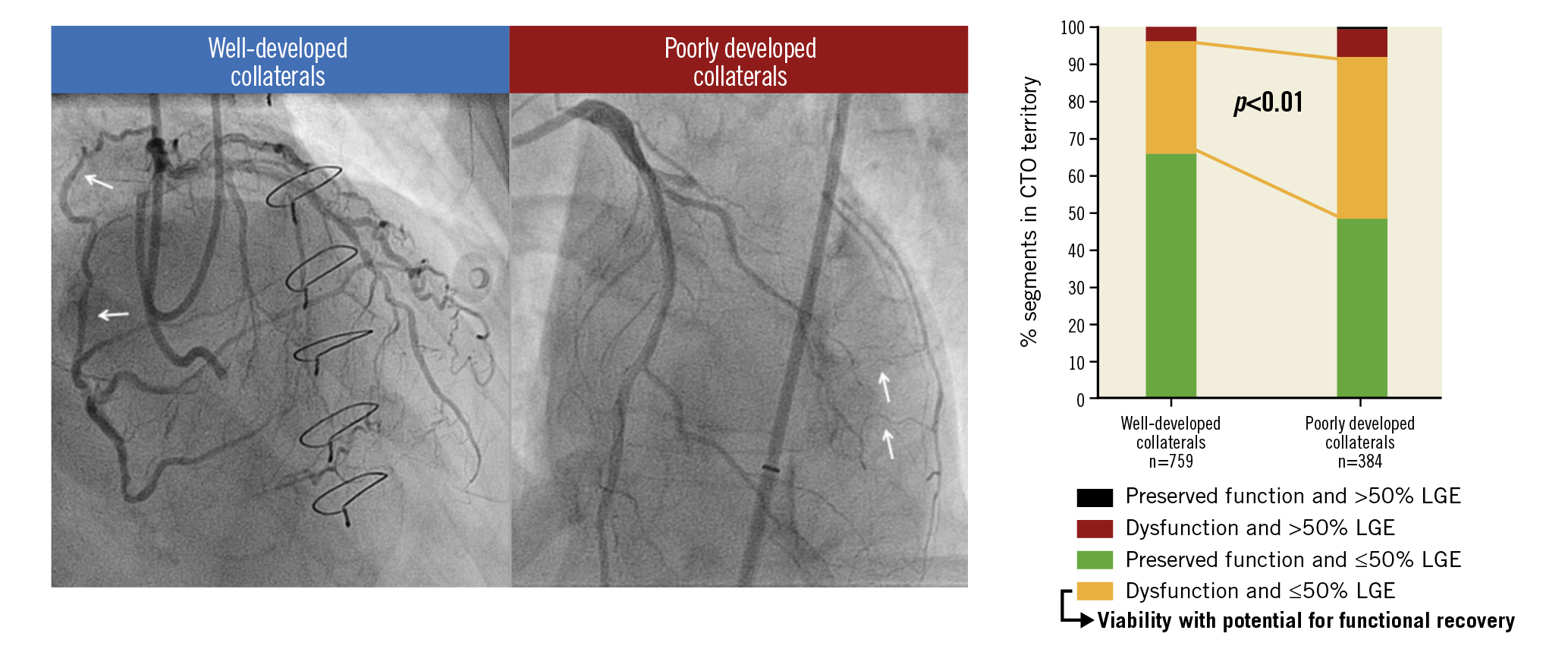
Visual summary. CMR-derived viability with potential for functional recovery is regularly present in myocardium supplied by poorly developed collaterals in patients with a CTO.
Introduction
Randomised trials evaluating the benefit of chronic coronary total occlusion (CTO) percutaneous coronary intervention (PCI) in addition to medical therapy on patients’ health status and prognosis have shown conflicting results, leading to an ongoing debate regarding judicious patient selection for CTO revascularisation1,2. Guidelines advocate viability assessment, next to evaluation of invalidating symptoms and ischaemia detection, to serve as one of the pillars in patient selection for CTO revascularisation3. A hampered regional and global functional recovery after CTO PCI in prior studies may be due to a lack of viability (dysfunctional but viable myocardium) demonstration before revascularisation4,5. Although there is a paucity of data, it has been hypothesised that the degree of collateral development on invasive coronary angiography correlates with the extent of myocardial infarction (MI) and viability6,7. The aim of the present study was to evaluate the association between coronary collaterals and viability as defined by cardiac magnetic resonance imaging (CMR) in patients with a CTO.
Methods
STUDY DESIGN AND PARTICIPANTS
Patients presenting with a CTO and evaluated with CMR between 2013 and 2018 in a dedicated CTO PCI centre (Amsterdam University Medical Center, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands) were prospectively recruited. Exclusion criteria were a non-occluded bypass graft on the CTO artery, signs of microvascular obstruction or non-ischaemic cardiomyopathy on CMR, and insufficient image quality for both cine and late gadolinium enhancement (LGE) analysis. Angiographic complexity and location of the CTO were both not reasons to exclude patients from evaluation by CMR. A documented history of MI was defined as Q-wave MI or non-Q-wave MI depending on the presence of pathological Q-waves on the electrocardiogram. The study was approved by the institutional Medical Ethics Review Committee and all patients provided written informed consent.
ANGIOGRAPHIC CHARACTERISTICS
Angiographic characteristics on invasive coronary angiography were scored by an expert observer (S.P. Schumacher) using a monoplane cardiovascular X-ray system (Allura Xper FD 10/10; Philips Healthcare, Best, the Netherlands). A CTO was defined as a luminal occlusion in a native coronary artery for an estimated time of ≥3 months with no or minimal contrast penetration through the lesion (Thrombolysis In Myocardial Infarction [TIMI] flow grade 0 or 1). Collaterals supplying the vascular territory of the CTO were graded according to the collateral connection (CC) score as no visible connection (CC0), thread-like connection (CC1) or side branch-like connection (CC2)8. The Rentrop gradation for collateral flow was scored as no contrast filling of collaterals (grade 0), filling of collaterals without epicardial filling of the CTO artery (grade 1), partial epicardial filling of the CTO artery (grade 2), and complete epicardial filling of the CTO artery (grade 3)8. A concomitant CC2 score and Rentrop grade 3 defined the presence of well-developed (WD) collaterals in a patient, whereas collaterals were considered poorly developed (PD) if the CC score was <2 or the Rentrop grade <37.
CMR IMAGING ACQUISITION AND ANALYSIS
All CMR images were obtained on a 1.5-T scanner (MAGNETOM® Avanto; Siemens Healthineers, Erlangen, Germany). A detailed description of acquisition techniques and CMR parameters is provided in Supplementary Appendix 1. Image analysis was performed by an experienced observer (H. Everaars) blinded to clinical and angiographic data, using commercially available software (QMass® 7.6; Medis Medical Imaging Systems, Leiden, the Netherlands). Left ventricle (LV) volumes and ejection fraction (LVEF) were calculated from cine images. In addition, the LV was divided into 16 segments (true apex not included) and vascular territories according to the American Heart Association (AHA) segmentation model, accounting for coronary dominance9. Segmental wall thickening (SWT), defined as absolute difference between end-diastolic and end-systolic wall thickness, was calculated from short-axis cine images for each segment and as a mean for the entire CTO territory. Dysfunctional myocardium was defined as SWT <3 mm10. Infarct size was calculated from LGE images and was expressed as a percentage of the LV. Additionally, percentage scar was calculated for each segment and as a mean for the entire CTO territory, in which 0%, ≤50% and >50% LGE defined absence of, limited and extensive scar, respectively11. Previously, interobserver and intraobserver variabilities of these SWT and LV scar analyses from the same institute were reported to be 0.1±0.7 and 0.0±0.4 mm and 1.2±4.1 g and 0.8±1.6 g12. As representative of myocardium with potential to recover functionally after revascularisation, viability was defined as dysfunctional myocardium without extensive scar6,11.
STATISTICAL ANALYSIS
Normally distributed data are presented as mean±standard deviation and compared using the independent samples t-test. Non-normally distributed continuous data are presented as median (interquartile range) and compared using the Mann-Whitney U test. Categorical data are presented as numbers (percentages) and were analysed using Fisher’s exact test. The proportions of CTO segments with no or limited scar, dysfunction or viability were compared between groups using generalised estimating equations (GEE) for binary data. A logistic link was used together with an unstructured working correlation matrix to account for possible dependency between outcomes measured in segments in the CTO territory of the same individual. When the overall effect for comparing ≥2 groups was significant, Bonferroni correction was applied for post hoc pairwise comparisons between groups. To test whether the proportion of segments with viability was different between patients with WD and PD collaterals and whether differences depended on subgroups based on diagnosis of prior MI in the CTO territory, we first fitted a GEE model with viability (yes/no) as the outcome and degree of collateral development (WD or PD), subgroup, and their two-way interaction as independent variables. A single overall GEE model was used to ensure that the same working correlation matrix estimate (based on all vessels) was used for all comparisons. Post hoc tests based on this GEE model were then used to check for differences between WD and PD collaterals within each of the subgroups based on prior MI diagnosis. A Bonferroni correction was applied to allow for separate comparisons between the four subgroups. A level of p<0.05 was considered significant. Statistical analyses were performed using SPSS software, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
PATIENT POPULATION
A total of 218 out of 250 consecutive patients with a CTO and evaluated by CMR were included for analysis. Sixteen patients were excluded because of a non-occluded bypass graft on the CTO artery, seven because of signs of non-ischaemic cardiomyopathy and five due to the presence of microvascular obstruction on CMR. In four patients, both cine and LGE images were of insufficient quality. Baseline clinical and angiographic characteristics of patients with WD (N=146, 67%) and PD (N=72, 33%) collaterals are presented in Table 1, Supplementary Table 1 and Supplementary Table 2. The intraobserver and interobserver agreement of angiographic classification of collaterals to WD or PD in 44 (20%) randomly chosen patients was 86% (Cohen’s kappa=0.65, 95% CI: 0.40-0.89) and 80% (Cohen’s kappa=0.57, 95% CI: 0.33-0.81), respectively.
RELATIONSHIPS BETWEEN MYOCARDIAL INFARCTION, FUNCTION AND COLLATERALS
In 147 patients without documented history of MI in the CTO territory, LGE analysis demonstrated myocardial scar in 112 (76%) CTO territories. In total, 183 (84%) patients had myocardial scar in the CTO region, with no difference in the prevalence of scar between patients with WD and PD collaterals (122 [84%] vs 61 [85%] patients, p=1.00). Median percentage scar tissue in the CTO territory, however, was lower in patients with WD collaterals (p=0.048) (Figure 1A). A normal LV function (LVEF >55%), mild dysfunction (LVEF >40-55%), moderate dysfunction (LVEF >30-40%) and severe dysfunction (LVEF ≤30) was observed in 62 (29%), 104 (48%), 29 (13%), and 21 (10%) patients, respectively. Mean LVEF was higher and LV end-diastolic and end-systolic volumes were smaller in patients with WD collaterals compared to patients with PD collaterals (p=0.02, p=0.047 and p=0.03, respectively) (Table 2). Mean SWT in the CTO territory was higher in patients with WD collaterals (p<0.01) (Figure 1B). Patients with multivessel disease had more scar tissue, larger LV volumes and worse LV function (Supplementary Table 3) compared to patients with single-vessel disease. Case examples of patients with WD and PD collaterals are shown in Figure 2. The amount of scar tissue in the LV and CTO territory was inversely correlated with LVEF and mean SWT in the CTO territory (Figure 3A, Figure 3B).
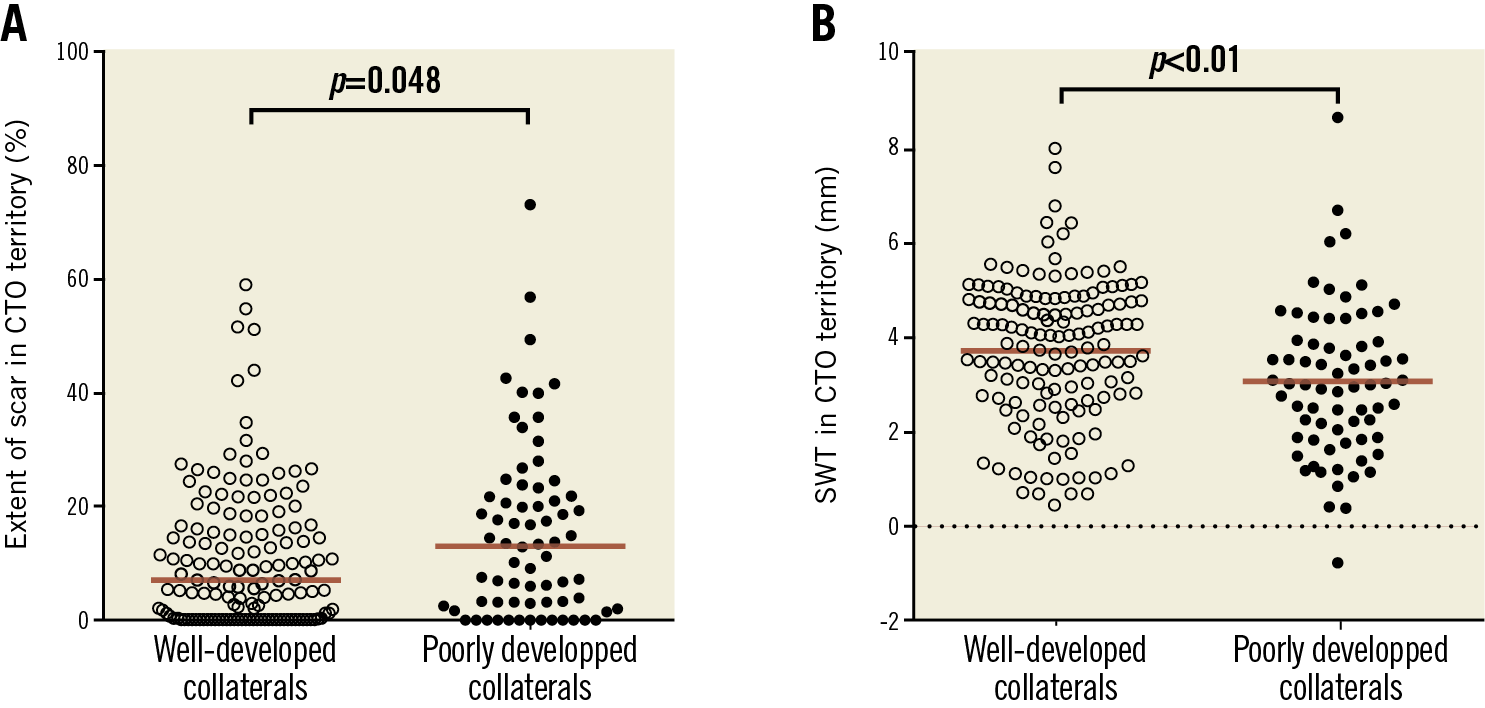
Figure 1. Median percentage scar tissue (A) and mean SWT (B) in the CTO territory stratified according to collateral status. Cine and LGE analysis could not be performed due to insufficient image quality in two and three patients, respectively. Red lines represent means. CTO: chronic coronary total occlusion; LGE: late gadolinium enhancement; SWT: segmental wall thickening
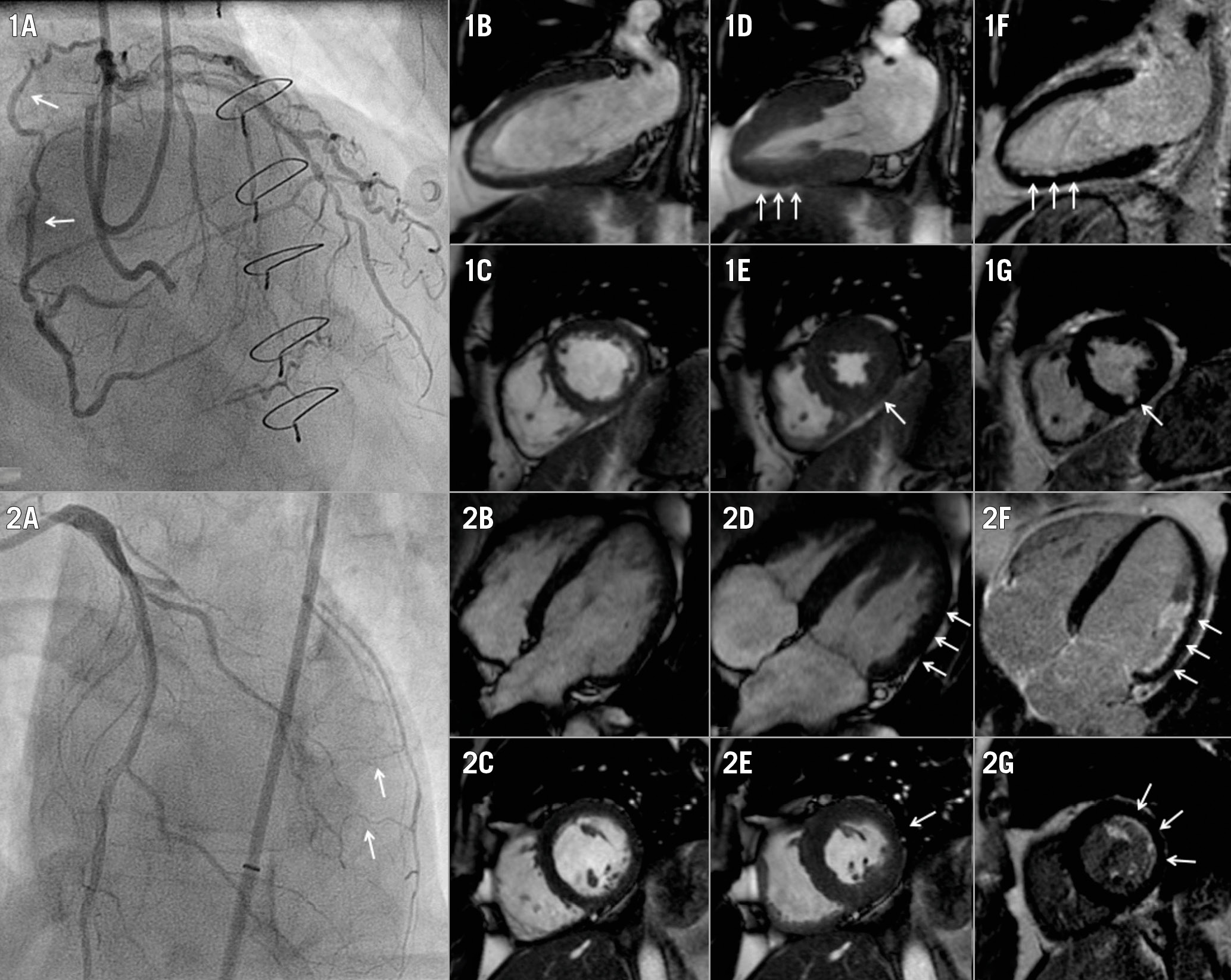
Figure 2. Case examples including patients with WD and PD collaterals. 1A) A WD collateral (arrow) supplies the vascular territory of a CTO in the right coronary artery. Two-chamber and short-axis views at end-diastolic (1B, 1C) and end-systolic (1D, 1E, arrows) phases show mild hypokinesia of the inferior wall, in the presence of limited subendocardial scar (arrows) detected with LGE (1F, 1G). 2A) PD collaterals supply the vascular territory of a CTO in the left circumflex artery. Four-chamber and short-axis views during end-diastolic (2B, 2C) and end-systolic (2D, 2E) phases display moderate hypokinesia (arrows) of the lateral wall, while LGE (2F, 2G) demonstrates a considerable (but still limited) amount of subendocardial scar (arrows). CTO: chronic coronary total occlusion; LGE: late gadolinium enhancement; PD: poorly developed; WD; well-developed
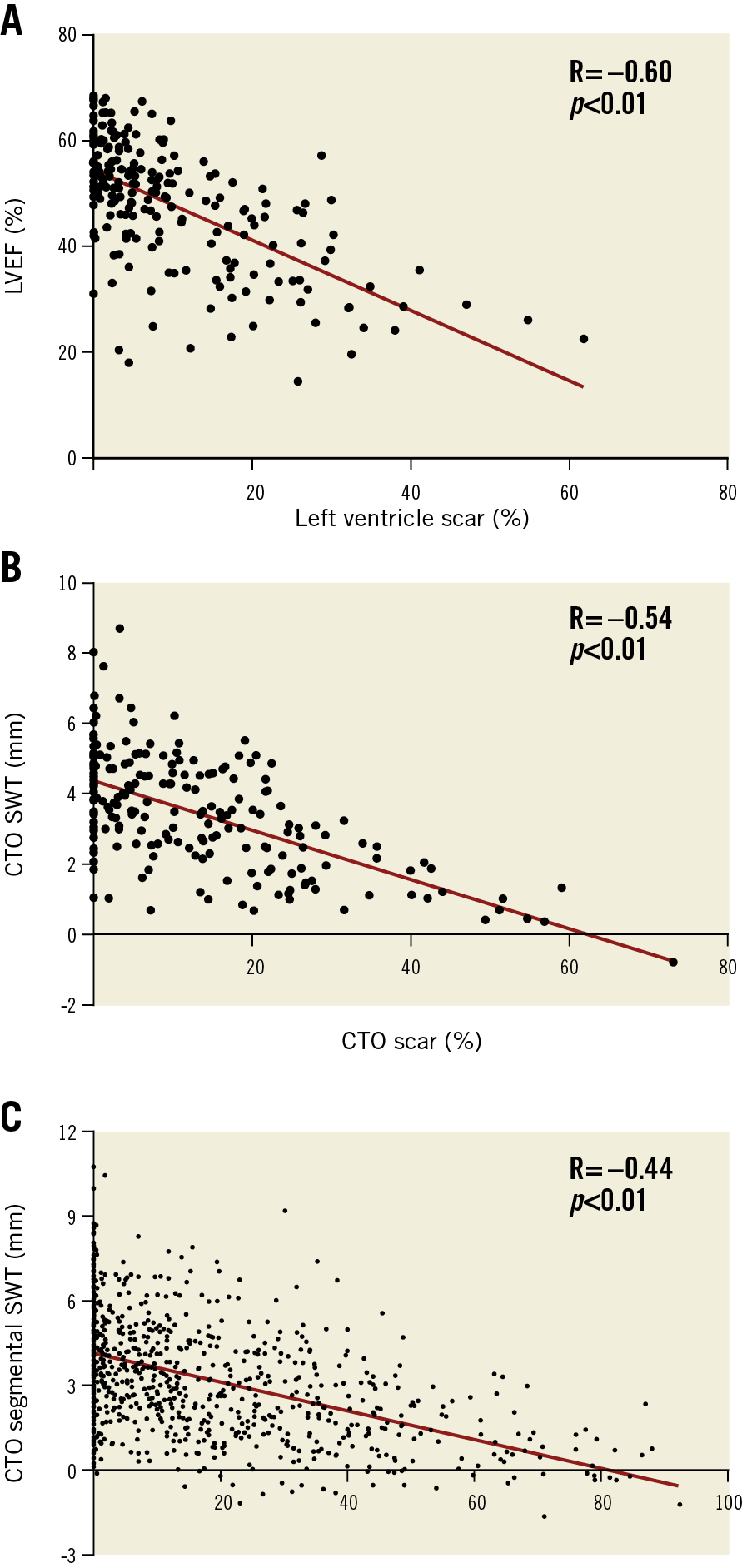
Figure 3. The correlation between left ventricular percentage scar and ejection fraction (A), and correlations between percentage scar tissue and SWT per CTO territory (B) and per segment in the CTO territory (C). CTO: chronic coronary total occlusion; LVEF: left ventricular ejection fraction; SWT: segmental wall thickening
MYOCARDIAL SCAR, DYSFUNCTION AND VIABILITY IN CTO TERRITORY-RELATED SEGMENTS
Absence of or ≤50% LGE was observed in 1,085 out of 1,143 (95%) segments in the CTO territory. Dysfunctional myocardium was present in 455 (40%) segments, with the other 688 (60%) demonstrating preserved function. Extent of scar and SWT in CTO segments were inversely correlated (Figure 3C). Viability, represented by dysfunctional myocardium without extensive scar, was present in 399 (35%) CTO segments. As shown in Figure 4A, Figure 4B, and Supplementary Table 4, CTO-related segments were analysed in all patients and in patients stratified according to documented CTO vessel-related history of MI and presence of scar in the CTO territory on CMR. In patients with WD collaterals, the prevalence of no or limited scar was higher compared to patients with PD collaterals (731 out of 759 [96%] vs 354 out of 384 [92%] segments, p<0.01), although differences were small. Myocardium was dysfunctional in a substantially higher number of segments in patients with PD collaterals compared to patients with WD collaterals (196 out of 384 [51%] vs 259 out of 759 [34%] segments, p<0.01). As a consequence, viability was present in more segments among patients with PD collaterals compared to patients with WD collaterals (168 out of 384 [44%] vs 231 out of 759 [30%] segments, p<0.01) (Figure 4C, Figure 4D). In addition, more CTO segments were dysfunctional in patients with multivessel disease compared to patients with single-vessel disease, leading to a higher number of segments with viability (Supplementary Table 3).
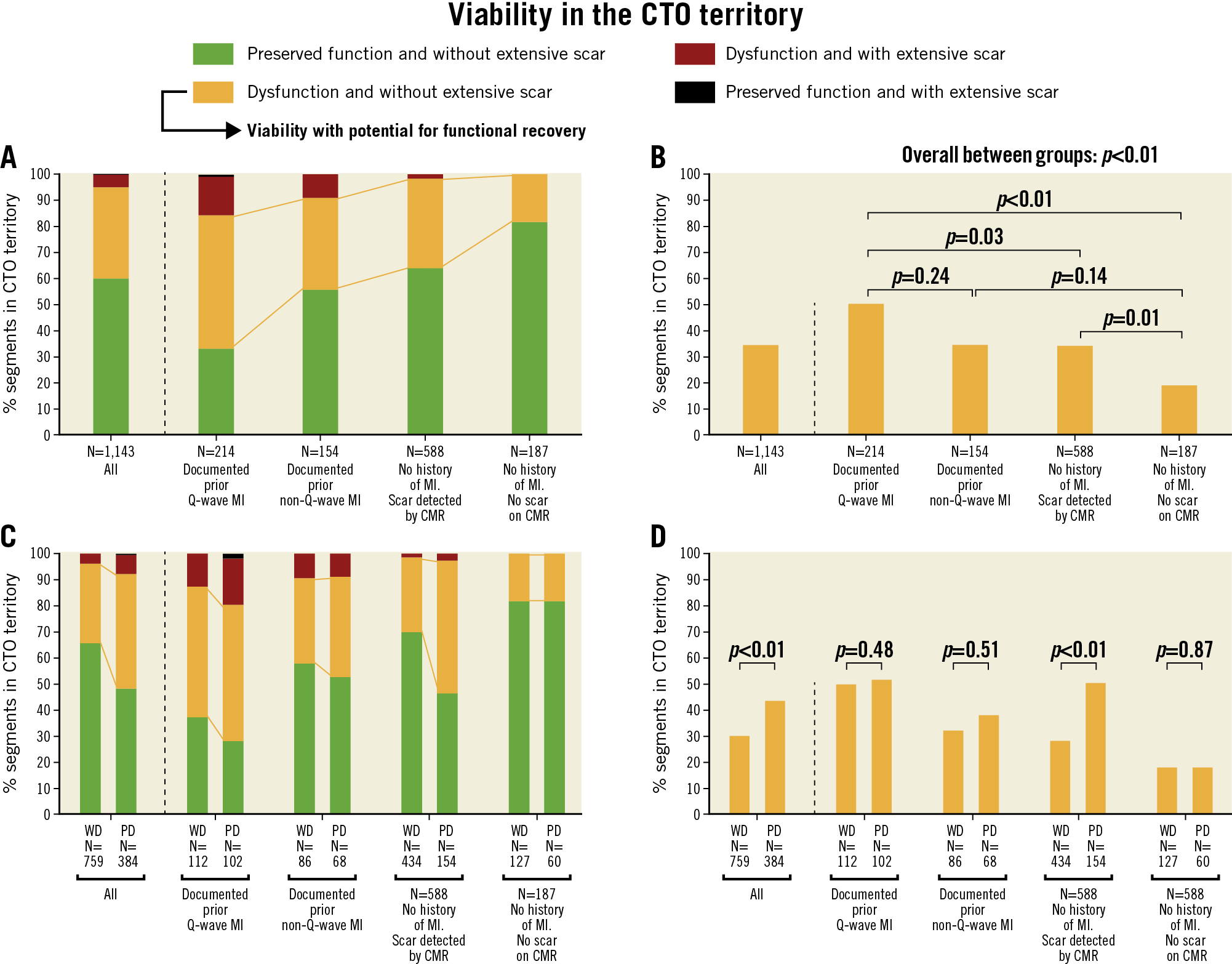
Figure 4. Distribution of CTO segments according to myocardial contractility and (non-)extensive scar formation. Numbers below the horizontal axis represent number of segments. A) CTO segments in all patients and in subgroups of patients stratified according to documented CTO vessel-related history of MI and presence (or absence) of scar in the CTO territory on CMR. B) Percentage of segments demonstrating viability with potential for functional recovery, compared among subgroups. C) Distribution of CTO segments according to collateral status. D) Percentage of segments demonstrating viability with potential for functional recovery compared between patients with WD collaterals and those with PD collaterals. CMR: cardiac magnetic resonance; CTO: chronic coronary toatal occlusion; MI: myocardial infarction; PD: poorly developed; WD: well-developed
Discussion
The present study is the largest to date evaluating the extent of myocardial infarction, function and viability assessed with CMR in patients with a CTO. The findings were additionally related to the presence of WD and PD collaterals on invasive coronary angiography. The infarcted area in the CTO territory was generally limited, with extensive scar observed in only 5% of CTO segments. Well-developed collaterals were associated with less scar and more preserved function in myocardium subtended by a CTO. Myocardial viability in the CTO territory with potential to recover functionally after revascularisation, in this study represented by dysfunctional myocardium without extensive scar, was more extensive in patients with PD collaterals.
DOCUMENTED HISTORY OF MI AND SCAR TISSUE ON CONTRAST-ENHANCED CMR
Choi et al previously demonstrated that LV scar tissue is present in a much larger proportion (86%) of patients with a CTO than clinically recognised7. The current study focused primarily on LGE quantification specifically in the CTO territory and revealed myocardial scar in the CTO region in 76% of patients without a documented CTO vessel-related history of MI. However, infarct sizes were often small, with 95% of CTO segments demonstrating no or limited scar tissue. In clinical practice, it is regularly assumed that a history of MI related to the CTO territory, especially in the presence of pathological Q-waves, excludes myocardium without transmural scar. This assumption seems to be inappropriate in the vast majority of patients according to this study.
MYOCARDIAL INFARCTION AND FUNCTION RELATED TO THE DEGREE OF COLLATERAL DEVELOPMENT
A previous study showed that infarct size after unsuccessful thrombolysis after STEMI was reduced in patients in whom collaterals that supply the infarct-related artery immediately could be visualised on invasive angiography13. Wustmann et al additionally demonstrated that the immediate well-functioning of the collateral system after brief vascular occlusion, as invasively measured with the collateral flow index, differs to a great extent between individuals with one fifth showing no signs of ischaemia14. These results raise the question as to whether collateral systems with a comparable angiographic appearance in patients with a CTO could have had variable protective effects on myocardial contractility in the past, especially if the CTO lesion developed from an untreated acute thrombotic event. In addition, collateral blood supply to myocardium subtended by a CTO could hypothetically be limited in patients with multivessel disease due to obstructive disease in another coronary artery proximal to the origin of the collateral. Nevertheless, when a CTO is depicted by diagnostic angiography in clinical practice, a protective role is regularly allocated to WD collaterals supplying a CTO territory, while a lack of myocardial preservation is attributed to PD collaterals. These assertions are substantiated by the present study, which demonstrated that patients with WD collaterals had less myocardial scar and more retained myocardial function in the CTO territory, consistent with a few smaller studies6,7. However, the present study additionally suggests that stratification by collateral status in the individual patient has limited value since regional infarct size was small and myocardial function preserved in the majority of patients with PD collaterals as well. These findings are in line with prior results by Werner et al, who observed that not the invasively measured collateral function but a lower microvascular resistance in myocardium subtended by a CTO was associated with regional functional improvement after CTO PCI15.
VIABILITY DETECTION AND POTENTIAL FOR FUNCTIONAL RECOVERY
The randomised REVASC trial and a recent meta-analysis including 34 observational studies showed a mean increase in LVEF of <5% after successful CTO revascularisation4,5. The clinical relevance of this modest functional improvement is questionable, especially considering recently published randomised trials showing no superiority in patients’ prognosis after CTO PCI compared to medical therapy1,2. The results of these trials suggest that invalidating symptoms may be considered the primary indication to refer a patient for CTO PCI. In contrast, a few observational studies demonstrated substantial improvements in LVEF if viability demonstration was included in the clinical decision making16,17. A prerequisite for viability is the presence of dysfunctional “hibernating” myocardium. Historically, hibernation is defined as chronic dysfunctional myocardium during a state of chronic hypoperfusion, although some prior data suggested the occurrence of repeated episodes of ischaemia rather than chronic hypoperfusion to be the cause of chronic regional wall motion abnormalities in collateral-dependent non-infarcted myocardium18. Dysfunctional myocardium suffering from chronic hypoperfusion or repeated episodes of ischaemia is expected to improve functionally after revascularisation18. In contrast, the presence of preserved contractility or extensive myocardial fibrosis before revascularisation can result in no or limited functional recovery11,17. The current gold standard for myocardial hibernation and viability detection is fluorine-18-deoxyglucose positron emission tomography (FDG-PET), which is often performed in conjunction with myocardial perfusion imaging to evaluate the presence of perfusion-metabolism mismatches. The choice for CMR as viability test from several non-invasive imaging modalities in the current study was based on local availability and expertise in this single centre. CMR-derived viability was represented by baseline dysfunctional myocardium without extensive scar and was scored per segment, leading to a more detailed indication of the extent of viability as compared to a per-vessel analysis. Viability was observed in 35% of CTO segments which equals two segments in a standardised vascular territory (consisting of five or six segments) according to the 17-segment model9. The relatively preserved LV function and limited amount of viability in the CTO territory substantiate the overall modest improvement in LVEF after CTO PCI in most studies4. In addition, a correlation was found between myocardial dysfunction and extent of scar tissue. Consequently, it cannot be genuinely distinguished whether the increased myocardial dysfunction (and viability) observed in CTO segments in patients with a prior Q-wave MI or PD collaterals is a marker of enhanced hibernating myocardium or a repercussion of augmented but still limited scar formation. Yet, it should be emphasised that neither PD collaterals nor a documented history of MI exclude viability according to the findings in the present study and should not be the reason to defer a patient from consideration for CTO revascularisation. Also in these patients, clinical decision making should take place after careful evaluation of all information regarding symptom severity, viability, and ischaemia, as advocated in current guidelines3.
Limitations
The patients in this study represent a single-centre population over the full LVEF range. Extent of scar tissue and dysfunction may therefore be less compared to prior studies with more stringent patient selection for viability assessment. The CMR analysis lacked myocardial perfusion imaging or dobutamine stress testing and therefore cannot genuinely indicate chronic hypoperfusion and hibernating myocardium. However, a prior study showed that current CMR analyses for myocardial viability assessment have high accuracy as referenced by FDG-PET19. Any patient selection bias by referring physicians from other centres because of the angiographic appearance or location of the CTO lesion cannot be ruled out. The intraobserver and interobserver agreements of visual collateral classification were modest, reflecting the difficulty to grade collaterals qualitatively. Invasive assessment of collateral function was not performed and could aid in exploring relationships between collateral function and myocardial viability. Coronary anatomy is known to vary, and subtle discordances with standardised myocardial segmentation cannot be excluded.
Conclusions
Although myocardial scar is commonly observed in patients with a CTO, infarct sizes are generally limited in the CTO territory. Well-developed collaterals are associated with less scar and more preserved function in myocardium subtended by a CTO. The extent of myocardial viability with potential for functional recovery in patients with a CTO seems to be modest; however, neither PD collaterals nor a CTO vessel-related history of MI exclude the presence of viability. Neither PD collaterals nor a history of MI related to the CTO territory should be a reason to defer a patient from consideration for CTO revascularisation.
|
Impact on daily practice In the present study, the infarcted area in myocardium subtended by a CTO was generally limited, even in patients with a documented history of MI. Although differences were small, well-developed collaterals were associated with less myocardial scar and enhanced preserved function. However, myocardial viability was regularly present in patients with poorly developed collaterals as well. These results show that patients with a CTO should not be deferred from consideration for CTO revascularisation based on the presence of poorly developed collaterals or a CTO artery-related history of MI. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

