
Interventional cardiologists are often assumed to be obsessed with the technicality of their work, just focused on performing perfect interventions that might be clinically unnecessary. Two studies in this issue of EuroIntervention come from an internationally renowned Dutch group of experts in the most difficult field of percutaneous coronary intervention (PCI), chronic total occlusion (CTO) revascularisation. Few other groups, including the best operators from Japan, can claim a 95% success rate in the last five years, as obtained in the patients treated in these two studies1,2. Good operators but good doctors as well, they were able to perform a complete clinical, anatomical and functional assessment of all their consecutive patients considered for CTO recanalisation.
In this single-centre study of nearly 200 CTO patients, two different techniques were used, cardiac magnetic resonance imaging (MRI) with late gadolinium to assess pre-intervention viability and positron emission tomography (PET) perfusion with labelled 02 to study ischaemia. In the first study report the assessment with MRI was performed only before interventions and at rest1; in the second study report serial PET acquisitions at rest and after hyperaemia were obtained before and three months after successful CTO PCI2. These two different studies with sophisticated techniques lead to very simple complementary messages. The MRI study shows that the majority of patients considered for CTO recanalisation, with or without history or signs of previous myocardial infarction, have some myocardial damage but not to the extent to make recanalisation futile. The PET study shows that ischaemia is present in all patients irrespective of the quality of collaterals present, with a major improvement and near-normalisation after treatment in all patients, greater in patients with large perfusion deficits (Figure 1).
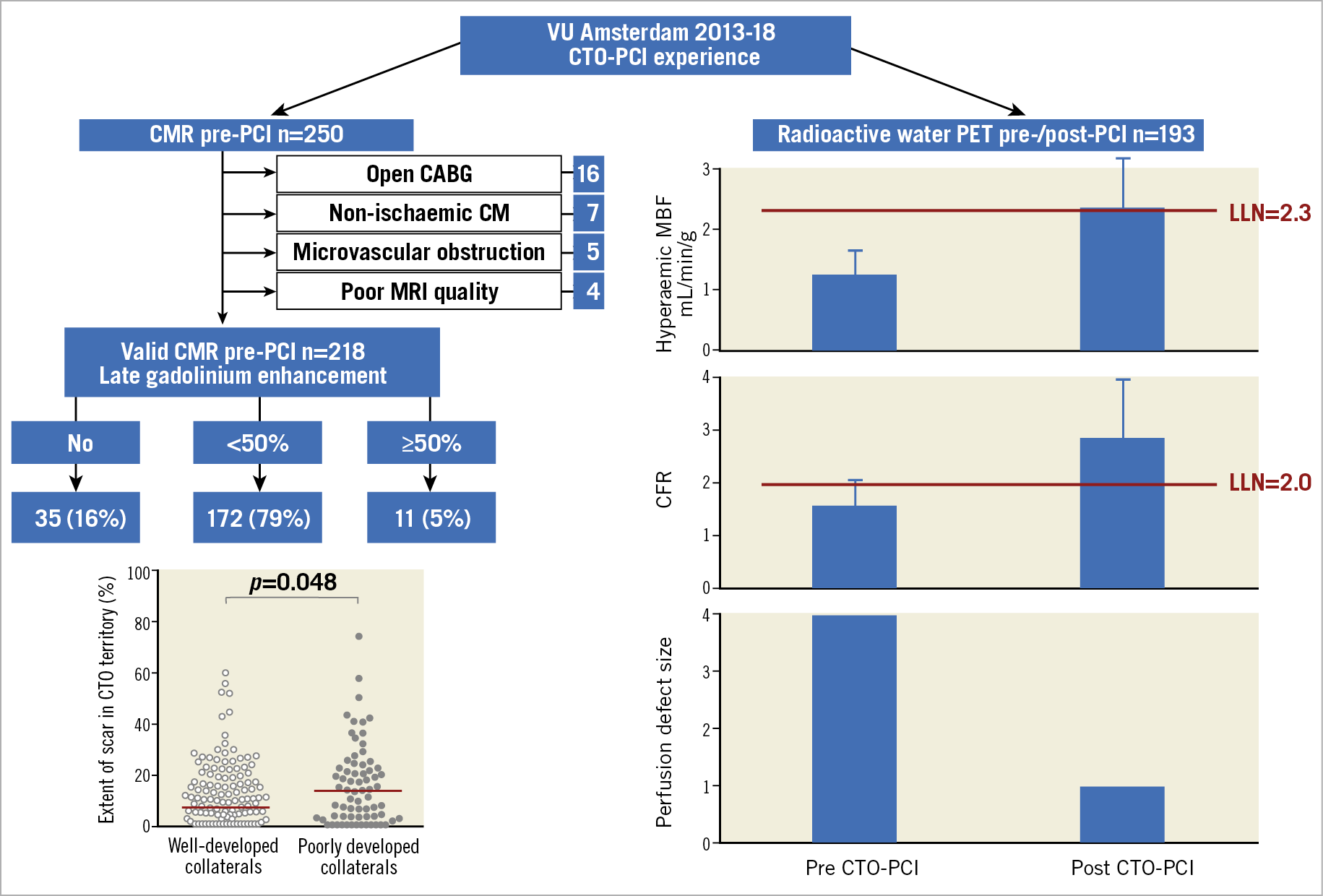
Figure 1. Viability and ischaemia in the CTO territory detected with CMR and PET. CABG: coronary artery bypass graft; CFR: coronary flow reserve; CM: cardiomyopathy; CMR: cardiac magnetic resonance; LLN: low limit of normal; MBF: myocardial blood flow; MRI: magnetic resonance imaging; PCI: percutaneous coronary intervention; PET: positron emission tomography; VU: Vrije University
Both findings deserve a comment. In some areas (carotid, lower limbs) chronic atherosclerotic occlusion is a stable condition not requiring treatment. The terminal circulation of the coronary arterial tree and the uneven blood supply provided by coronary collaterals justify the presence of small patchy areas of subendocardial fibrosis in most patients, unexpected because of the lack of history of myocardial infarction (MI) and an often normal electrocardiogram (ECG) and echocardiogram. Conversely, very few patients considered for recanalisation of chronic occlusions present with a full thickness scar. In the Netherlands, 25 years after the pivotal experience of Zwolle, there is universal application of rapid mechanical revascularisation with primary angioplasty for patients presenting with acute chest pain. CTO patients may have had silent MI but with atypical mild symptoms that did not induce them to seek medical help. They probably already had collaterals developing in response to a slow process of progressive occlusion. This is the reason why the findings of the OAT trial3, focused on opening the occluded infarct-related artery between two and 30 days post STEMI, are not applicable to CTO patients where, as in this series, only 28% of patients have a previous history of MI in the CTO territory and 5% of patients have more than 50% late gadolinium enhancement (LGE). Also, these patients received CTO recanalisation, probably because the territory of distribution of the occluded artery was larger than the area of irreversible damage. Collaterals are important in order to maintain viability and, even with all the limitations of their qualitative assessment including poor reproducibility, this MRI study showed that well-developed collaterals with rapid complete filling of the occluded artery protect against myocardial fibrosis. Poorly developed collaterals, however, are not a reason for withdrawing recanalisation because they are also associated with a greater chance of having impaired wall motion (WM) with less than 50% LGE thickness, i.e., hibernation, with greater potential of WM improvement after successful PCI.
If well-developed collaterals preserve the myocardium, can they prevent ischaemia in the CTO territory and spare the use of a time-consuming risky procedure such as CTO PCI? Looking at the angiographic images of Figure 3 (panel A2 and panel B2) and the corresponding PET scans at rest and after adenosine, you already have an answer2. The posterior descending artery (PDA) appears well filled by a full channel collateral at the apex from the left anterior descending artery (LAD)2. The same artery is filled anterogradely when the proximal right coronary artery (RCA) is open but the corresponding PET images during hyperaemia are completely different. When the PDA was only filled by retrograde collaterals, absolute flow during hyperaemia was low, while flow normalised after recanalisation. The same poor flow before and major improvement after occurred in nearly all the 193 successfully treated CTO patients. Larger initial perfusion deficits were associated with a lower hyperaemic absolute blood flow before PCI but managed to catch up after PCI. The quality of collaterals (coronary collateral score 0/1 vs 2) made no difference to the severity of ischaemia pre-PCI and the completeness of normalisation after.
Do we need to perform MRI and PET before and after all CTO revascularisations? In most multidisciplinary Heart Team meetings I have attended in Italy or the UK, all clinical non-invasive cardiologists would raise their eyebrows if you recommended proceeding to a life-threatening CTO recanalisation without objective proof of ischaemia and viability. Still, based on the results of these studies, you may dismiss them as clinically irrelevant after having seen that nearly all patients listed for CTO have both viability and ischaemia confirmed, with improvement of ischaemia post PCI also in MI patients. Still, this is a highly selected group of very symptomatic patients, 74% of them with persistent stable angina under multiple antianginals and referred to a tertiary referral hospital often after failed procedures elsewhere. In patients with possible previous MI, especially if Q-waves or regional WM abnormalities are present, at least a basal MRI waiting 10 minutes for the redistribution phase after gadolinium is worthwhile before embarking on a complex procedure such as CTO PCI.
What about ischaemia testing? Based on these findings, it is probably irrelevant before angioplasty, but some may argue that it adds little discomfort and cost to repeat the MRI acquisition after adenosine or dobutamine4. The search for ischaemia, however, might be more helpful as a follow-up study after successful CTO. In this study, there is evidence of residual ischaemia in a number of patients three months after successful CTO PCI. Restenosis is rare so soon after second-generation DES implantation, but ischaemia may persist or develop after treatment with multiple stents that are not always optimally expanded at the end of a long procedure and with distal vessels that may remodel over time. The persistence of critical lesions distal to the stented segment or of large branches with impaired flow distal to the CTO may explain residual or recurrent symptoms and may deserve non-invasive ischaemia testing with a low threshold to repeat angiography and optimise the PCI results, if needed.
Ischaemia in more than two segments (>10% of total myocardium, present in 92% of these patients) is considered a valid indication to perform revascularisation to improve prognosis, also in patients with mild or no anginal symptoms. Can we expect a survival benefit from performing PCI in this cohort of CTO patients? Randomised CTO trials showed no survival benefit, but results were hampered by design bias (for instance, simultaneous revascularisation of CTO and non-CTO vessels in DECISION PCI5) but also unavoidable selection bias facilitating the randomisation of patients with mild symptoms and small occluded vessels6,7. Also in this all-comers group the chances of survival benefit would be very small. Only a minority of patients had evidence of reversible WM changes and anyway very few patients had an ejection fraction below 40%. When results of CTO recanalisation were assessed in patients with a low left ventricular ejection fraction (LVEF)8, an increase in EF that was statistically as well as clinically relevant was observed. In these patients, CTO recanalisation can stabilise borderline ischaemic zones and reduce malignant arrhythmias9. Even a careful reading of the 123 pages of the Supplementary Appendix of the ISCHEMIA trial10 does not reveal the number of CTO lesions treated in the 2,588 patients of the invasive arm but probably CTO and diffuse disease were the main anatomical causes that prevented revascularisation in the 16.3% of patients of the invasive group crossing over to optimal medical treatment only. ISCHEMIA has, however, one similarity with all the CTO randomised studies and most registries including these last two: LVEF was normal at 60% (IQR 55-65%), equal in the invasive and the conservative groups. Despite the confirmed large ischaemic burden that was a prerequisite for enrolment, ischaemic relief with revascularisation did not affect the cardiovascular death rate that was low and similar at 2.8% and 2.9% at two years, non-significant (ns)10. Prevention of spontaneous MI in the invasive arm of ISCHEMIA is still under great scrutiny but acute ischaemic events are less likely in the distribution of an already occluded artery. It is time to forget the “hard” endpoints and accept that demonstrating mortality/MI reductions is beyond the possibility of any feasible trial of CTO recanalisation. The aim of revascularisation must be symptomatic improvement, not a surrogate endpoint but a very valuable goal, achievable with successful CTO PCI, as confirmed in well conducted randomised trials6 and controlled registries11,12. As recommended in the most recent EuroCTO Club consensus document13, the most important selection criterion for CTO PCI does not come from sophisticated tests. We should listen carefully to our patients. Not everybody will immediately complain of unbearable angina symptoms, but functional limitation can be unmasked by questionnaires or cardiopulmonary tests that can also grade its severity objectively. Remember, CTO patients often complain of breathlessness or tiredness rather than angina, but the vast majority of them still improve after CTO PCI.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

