Abstract
Background: Revascularisation of a chronic total coronary occlusion (CTO) impacts the coronary physiology of the remote myocardial territory.
Aims: This study aimed to evaluate the intrinsic effect of CTO percutaneous coronary intervention (PCI) on changes in absolute perfusion in remote myocardium.
Methods: A total of 164 patients who underwent serial [15O]H2O positron emission tomography (PET) perfusion imaging at baseline and three months after successful single-vessel CTO PCI were included to evaluate changes in hyperaemic myocardial blood flow (hMBF) and coronary flow reserve (CFR) in the remote myocardium supplied by both non-target coronary arteries.
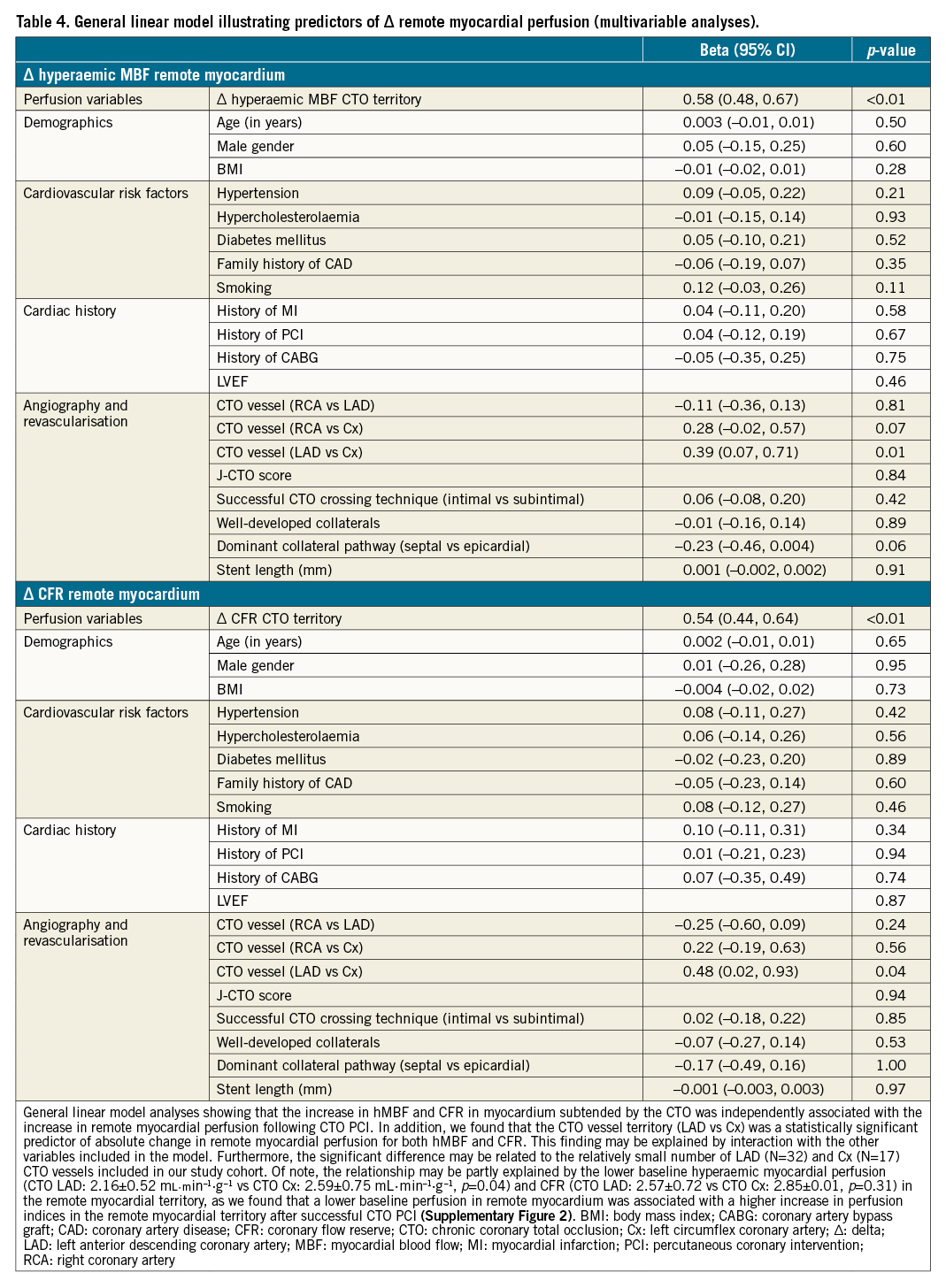
Results: Perfusion indices in CTO and remote myocardium showed a positive correlation before (resting MBF: r=0.84, hMBF: r=0.75, and CFR: r=0.77, p<0.01 for all) and after (resting MBF: r=0.87, hMBF: r=0.87, and CFR: r=0.81, p<0.01 for all) CTO PCI. Absolute increases in hMBF and CFR were observed in remote myocardium following CTO revascularisation (from 2.29±0.67 to 2.48±0.75 mL·min–1·g–1 and from 2.48±0.76 to 2.74±0.85, respectively, p<0.01 for both). Improvements in remote myocardial perfusion were largest in patients with a higher increase in hMBF (β 0.58, 95% CI: 0.48-0.67, p<0.01) and CFR (β 0.54, 95% CI: 0.44-0.64, p<0.01) in the CTO territory, independent of clinical, angiographic and procedural characteristics.
Conclusions: CTO revascularisation resulted in an increase in remote myocardial perfusion. Furthermore, the quantitative improvement in hMBF and CFR in the CTO territory was independently associated with the absolute perfusion increase in remote myocardial regions. As such, CTO PCI may have a favourable physiologic impact beyond the intended treated myocardium.
Introduction
Chronic total coronary occlusions (CTOs) have been reported in approximately 20% of patients with significant coronary artery disease (CAD) referred for invasive coronary angiography1. Myocardial ischaemia in the CTO territory is observed in the vast majority of patients, despite the presence of well-developed collaterals2. A growing body of evidence shows that successful CTO percutaneous coronary intervention (PCI) results in a reduction of ischaemic burden and is associated with improved quality of life34. Physiological evaluation is increasingly used in the selection of revascularisation strategies for obstructive lesions in coronary donor arteries supplying collaterals to the myocardium subtended by a CTO5. Distal flow characteristics of the CTO vessel are affected by microvascular function, the development and integrity of the collateral circulation, the effect of collateral steal, and obstructive disease in the collateral donor arteries678. Vice versa, the presence of a concomitant CTO results in alterations in coronary haemodynamics in remote myocardium subtended by the collateral donor coronary arteries5. Prior studies showed improvement in absolute myocardial perfusion in the myocardium subtended by a CTO and an increase in fractional flow reserve (FFR) in the CTO vessel after successful CTO PCI, whereas a concomitant immediate and subsequent further gradual decrease in collateral function was observed9101112. In addition, physiologic changes in collateral donor vessel flow velocity and pressure-derived estimations of coronary flow have been described following CTO PCI, largely attributable to collateral regression1314. To date, comprehensive studies evaluating coronary physiology in remote myocardium after CTO PCI have lacked quantitative perfusion assessment. The present study aimed to explore the intrinsic effect of CTO PCI on changes in absolute perfusion in remote myocardium as assessed by serial [15O]H2O positron emission tomography (PET) perfusion imaging.
Methods
Study design and participants
This study comprised prospectively recruited patients who underwent functional assessment with [15O]H2O PET perfusion imaging at baseline and approximately three months after successful single-vessel CTO PCI in the Amsterdam University Medical Center, Vrije Universiteit, between December 2013 and March 2019. The clinical indication for revascularisation was based on cardiac symptom burden, the extent of myocardial ischaemia, viability testing when indicated, and invasive coronary angiography. The decision to perform CTO revascularisation was left to the discretion of the Heart Team. Patients who underwent ischaemia detection using quantitative [15O]H2O PET perfusion imaging prior to referral for CTO PCI were screened for potential inclusion in the study. Patients in whom successful CTO revascularisation was performed were rescheduled for [15O]H2O PET imaging three months after the index procedure. Exclusion criteria comprised pregnancy and contraindications for the administration of adenosine. The flow chart of the study population has been described previously15. Patients included in the study cohort described by Schumacher et al15 were eligible for inclusion in the present study if they were successfully treated by single-vessel CTO PCI and additional revascularisation was not performed nor scheduled. The study complied with the Declaration of Helsinki and was approved by the institutional Medical Ethics Committee. All participants provided written informed consent.
Angiographic and procedural characteristics
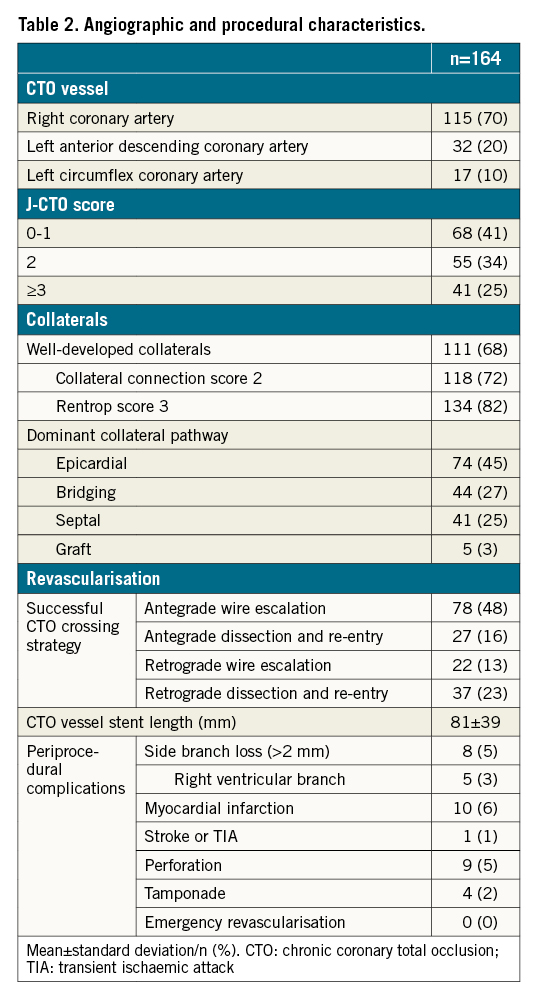
Angiographic and procedural characteristics were evaluated using a monoplane cardiovascular X-ray system (Allura Xper FD 10/10; Philips Healthcare). A CTO was defined as a luminal occlusion of a coronary artery with a documented or estimated duration of ≥3 months and no or minimal contrast penetration through the lesion on invasive coronary angiography (Thrombolysis In Myocardial Infarction [TIMI] flow grade 0-1)16. The extent of collateral development was assessed angiographically using the collateral connection (CC) score and Rentrop flow grade. Patients with a concomitant CC score 2 (branch-like connection) and Rentrop grade 3 (complete epicardial filling of the recipient CTO artery via collateral channels) were considered to have well-developed collaterals17. The angiography-derived Japanese (J)-CTO score was calculated for all CTO lesions and the operators applied the hybrid approach to achieve recanalisation of the CTO18. The CTO vessel was considered successfully revascularised if TIMI grade 3 flow was achieved and the residual diameter stenosis was <30%. Periprocedural myocardial infarction (MI) was scored following the Fourth Universal Definition of Myocardial Infarction19.
[15O]H2O PET ACQUISITION
[15O]H2O PET perfusion images were acquired on a hybrid PET/computed tomography (CT) scanner (Ingenuity TF 128; Philips Healthcare), as described previously20. Briefly, a dynamic emission scan was performed using 370 MBq of [15O]H2O during resting conditions and adenosine (140 µg·kg-1·min-1)-induced maximal vasodilator stress. Both resting and stress sequences were followed by a low-dose CT scan for scatter and attenuation correction. Parametric perfusion images were used for quantitative analysis to obtain the absolute myocardial blood flow (MBF) in mL·min-1·g-1 of perfusable tissue, using in-house developed software (Cardiac VUer)21. Resting MBF, hyperaemic MBF (hMBF) and coronary flow reserve (CFR), defined as the ratio between hMBF and resting MBF, were calculated for the myocardium subtended by the CTO lesion and the remote myocardial territory allocated according to the standardised vascular territories (right coronary artery, left anterior descending coronary artery and left circumflex coronary artery) of the 17-segment model of the American Heart Association (AHA)22. The myocardium supplied by the two non-target coronary arteries was defined as remote myocardium. The perfusion defect size in the CTO territory was calculated by adding up the segments within the CTO region in which hMBF was ≤2.3 ml·min-1·g-1 and <75% of the hMBF in a normal reference vascular territory420. A baseline perfusion defect size of ≥4 segments was classified as large4. A case example showing coronary angiograms and [15O]H2O PET perfusion images at baseline and after CTO PCI is presented in the Central illustration. To correct for the potential impact of overlapping adjacent vascular regions, analyses were repeated with remote myocardium excluding myocardial segments adjacent to the CTO region (Supplementary Figure 1). For all figures illustrating remote myocardial perfusion, a concomitant supplementary figure was included showing myocardial perfusion in the remote myocardial territory non-adjacent to the CTO region.
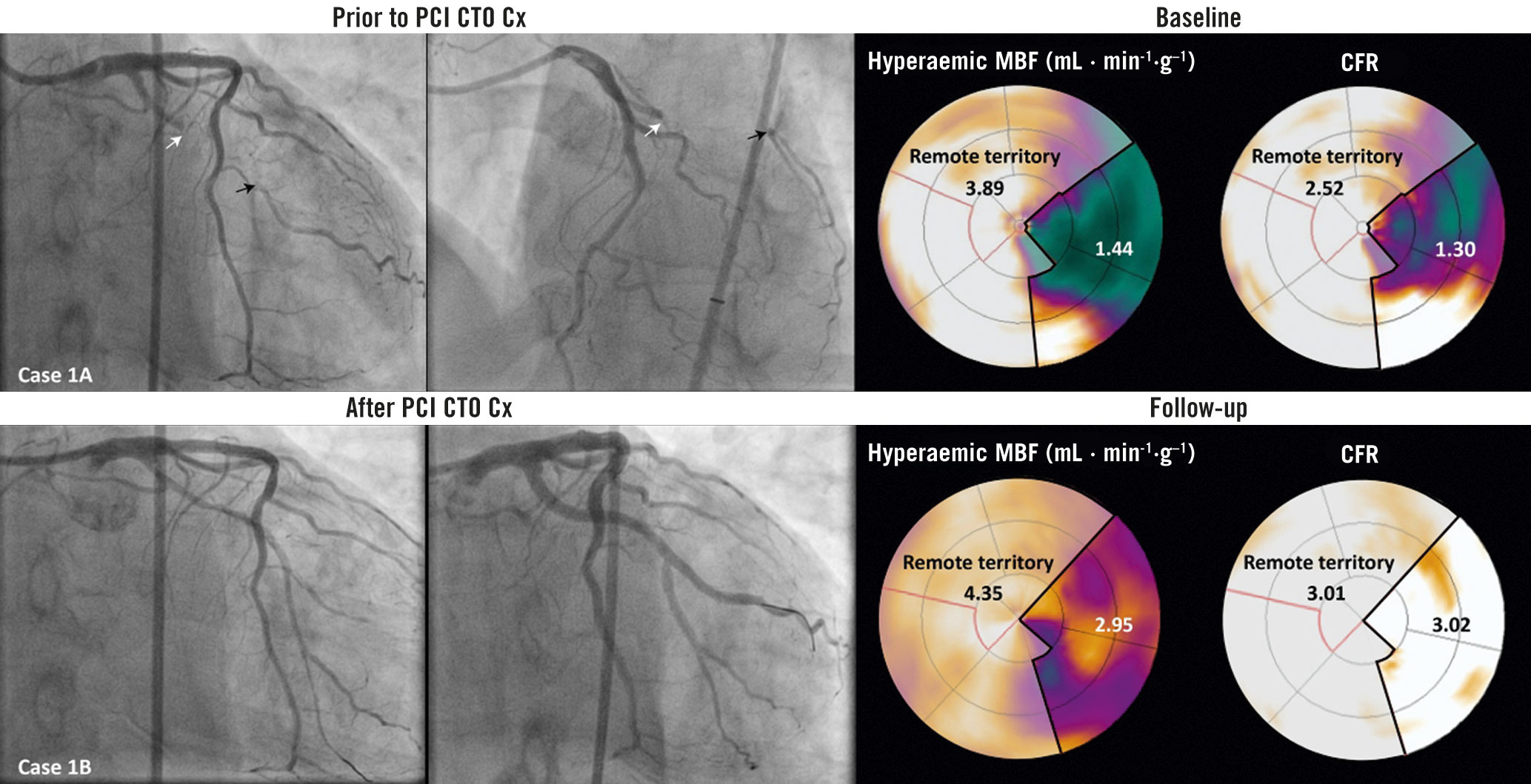
Central illustration. Coronary angiograms and [15O]H2O PET perfusion images illustrating the change in absolute remote myocardial perfusion following CTO PCI. A case example illustrating angiograms before and directly after successful recanalisation of a CTO in the Cx. [15O]H2O PET perfusion images of vasodilator stress flow and coronary flow reserve show absolute myocardial perfusion in the CTO and remote myocardial territory at baseline and follow-up PET imaging. Case 1A. Dual injection angiography images show a CTO of the Cx (white arrow). The black arrows indicate the well-developed collaterals (CC score 2 and Rentrop grade 3). hMBF and CFR in the CTO territory are lower compared to the remote myocardial territory. Case 1B. Angiography shows the circumflex coronary artery of the same patient during and directly after CTO revascularisation. Follow-up PET perfusion imaging shows improvements in hMBF and CFR in the CTO and remote myocardial territory. CC: collateral connection; CFR: coronary flow reserve; CTO: chronic total occlusion; Cx: circumflex coronary artery; hMBF: hyperaemic myocardial blood flow; PCI: percutaneous coronary intervention; PET: positron emission tomography
Statistical analysis
Categorical variables are presented as frequencies with percentages, whereas continuous variables are displayed as mean±standard deviation (SD) or median (interquartile range). Differences between normally distributed data were tested using the paired samples t-test for paired data, and the independent samples t-test for comparing two independent groups. Differences between continuous variables that were not normally distributed were analysed using the Wilcoxon signed-rank test for paired data or the Mann-Whitney U test for independent groups. Linear regression was used to assess the associations between absolute perfusion in the CTO and remote myocardium at baseline and after CTO revascularisation. Furthermore, a general linear model was fitted to evaluate the relationship between the absolute change in myocardial perfusion in the CTO territory and the remote myocardial territory after CTO revascularisation, adjusting for clinical, angiographic and procedural characteristics (age, gender, body mass index [BMI], hypertension, hypercholesterolaemia, diabetes mellitus, a positive family history of CAD, smoking status, prior cardiac history, CTO vascular territory, J-CTO score, CTO crossing technique, collateral development, the dominant collateral pathway and CTO vessel stent length) as potential confounders. Additionally, a general linear model was used to find predictors of change in perfusion indices in the remote myocardial territory following CTO PCI. All statistical analyses were performed using the SPSS software package, Version 26.0 (IBM).
Results
Study population
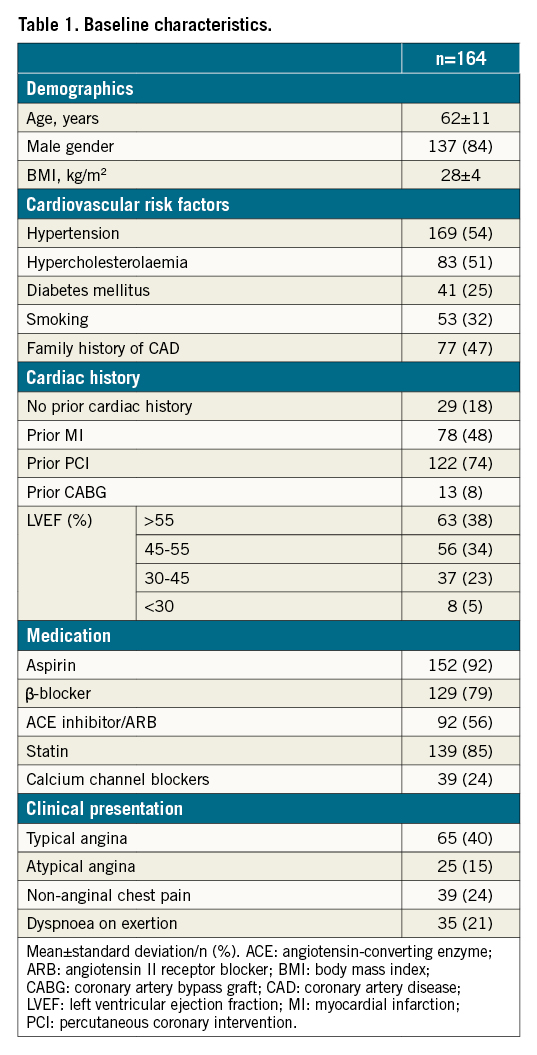
A total of 164 patients (mean age 62±11 years, 84% male) were included in the study (Table 1). The median interval between baseline PET imaging and CTO revascularisation was 40 (23-59) days, followed by a median interval of 101 (92-119) days until the follow-up PET perfusion scan was performed. Between CTO PCI and follow-up PET imaging, no cardiac events occurred and additional revascularisation was not performed. Angiographic and procedural characteristics are listed in Table 2. In 17 (10%) patients, a residual significant coronary stenosis remained after single-vessel CTO PCI. These lesions were triaged by the Heart Team and/or two experienced CTO operators (P. Knaapen/A. Nap). Additional revascularisation was not performed nor scheduled due to technical unfeasibility or an absent clinical indication for further revascularisation therapy, e.g., a limited myocardial territory subtended by the lesion. Supplementary Table 1 shows the residual coronary lesion location, the degree of coronary stenosis and its potential impact on coronary flow through the collateral circulation to the CTO territory in these patients. In five patients, a residual coronary lesion limited flow from the coronary collateral donor arteries to the myocardium subtended by the CTO prior to successful CTO revascularisation.
Correlation between CTO and remote myocardial territory
PET perfusion measurements before and after CTO PCI are shown in Table 3. Strong positive correlations were observed between resting MBF, hMBF and CFR in the CTO and remote myocardium, both before and after CTO PCI (Figure 1). Lower baseline resting MBF, hMBF and CFR values in the CTO myocardium were concurrent with lower perfusion measurements in the remote myocardial territory (resting MBF: r=0.84, p<0.01; hMBF: r=0.75, p<0.01 and CFR: r=0.77, p<0.01). Similar correlations were observed at follow-up PET imaging (r=0.87, p<0.01; r=0.87, p<0.01 and r=0.81, p<0.01 for resting MBF, hMBF and CFR, respectively).
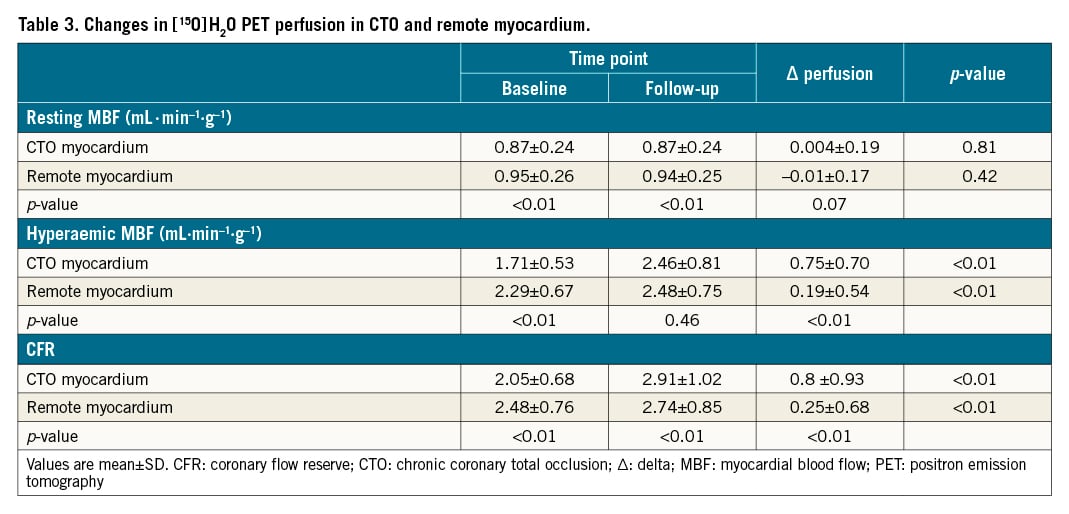
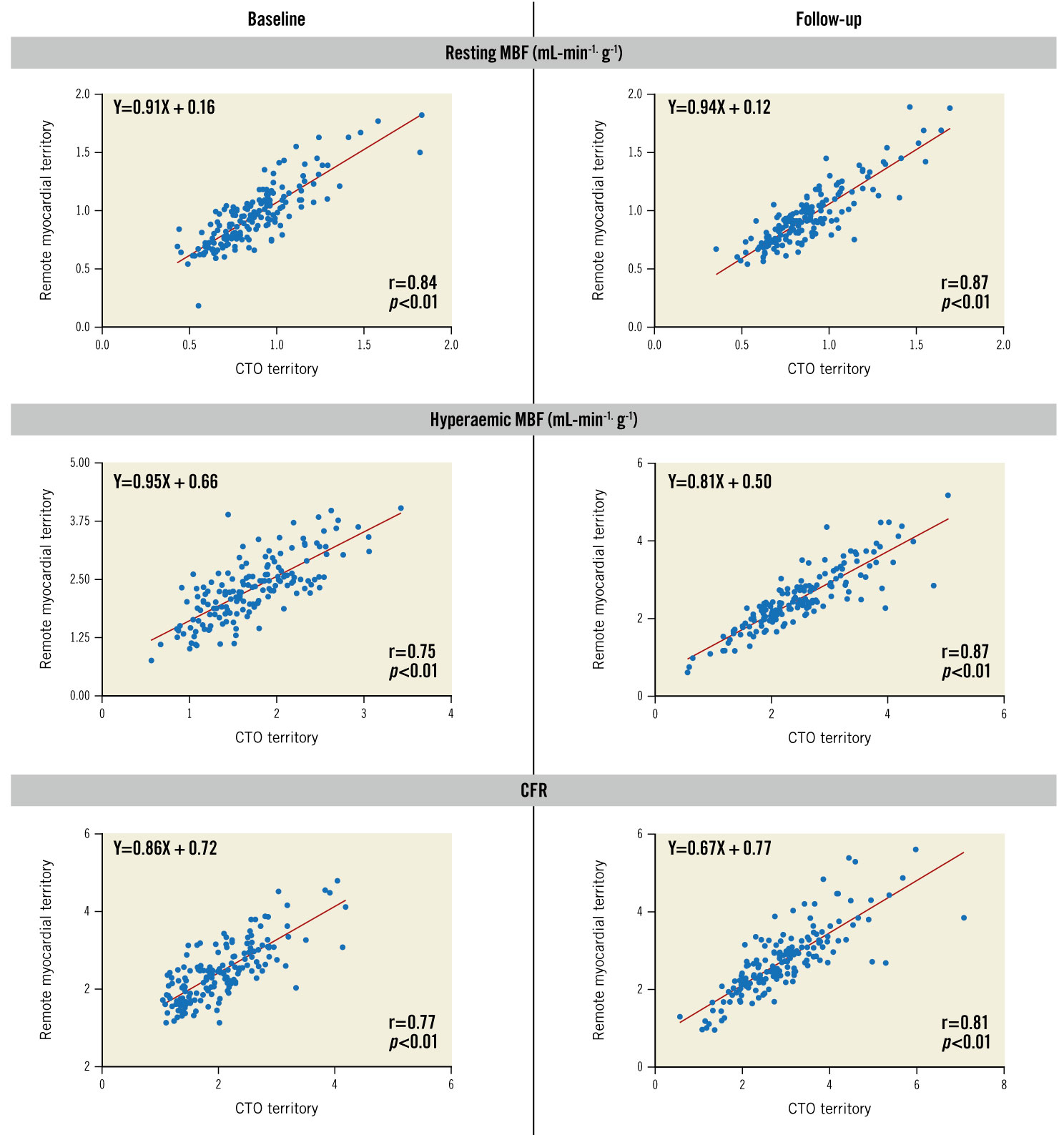
Figure 1. Association between perfusion indices in the CTO territory versus remote myocardial territory. A positive correlation was found between resting MBF, hMBF and CFR in the CTO and remote myocardial territory, both before and after CTO revascularisation. CFR: coronary flow reserve; CTO: chronic total occlusion; hMBF: hyperaemic myocardial blood flow; MBF: myocardial blood flow; PCI: percutaneous coronary intervention; PET: positron emission tomography
Increase in myocardial perfusion after CTO PCI
Resting MBF did not significantly change following CTO PCI (Table 3). In remote myocardium, hMBF (∆0.19±0.54 mL·min-1·g-1, p<0.01) and CFR (∆0.25±0.68, p<0.01) increased following CTO revascularisation. In the myocardium subtended by the CTO, perfusion increase between baseline and follow-up PET perfusion imaging was ∆0.75±0.70 mL·min-1·g-1 and ∆0.87±0.93 for hMBF and CFR, respectively (p<0.01 for both). Perfusion increase in the CTO myocardium was positively correlated with increase in remote myocardial perfusion (r=0.71, p<0.01 for hMBF and r=0.68, p<0.01 for CFR) (Figure 2). In a model adjusted for clinical, angiographic and procedural characteristics, the increase in perfusion indices in the CTO territory remained independently associated with the increase in remote myocardial perfusion (Table 4). We performed additional analyses to compare absolute myocardial perfusion indices between patients with and without a history of previous MI and found that the change in remote myocardial perfusion was similar in patients with a history of MI versus no MI for resting MBF (−0.02±0.18 mL·min-1·g-1 vs −0.01±0.16 mL·min-1·g-1, p=0.84), hMBF (0.16±0.49 mL·min-1·g-1 vs 0.22±0.58 mL·min-1·g-1, p=0.51) and CFR (0.23±0.65 vs 0.28±0.71, p=0.64) (Supplementary Table 2). Similarly, the change in myocardial perfusion indices in the remote myocardial regions did not differ in patients with a prior MI in the CTO territory compared to patients without a prior MI (resting MBF: −0.03±0.20 mL·min-1·g-1 vs −0.01±0.16 mL·min-1·g-1, p=0.62; hMBF: 0.19±0.56 mL·min-1·g-1 vs 0.22±0.58 mL·min-1·g-1, p=0.81 and CFR: 0.32±0.75 vs 0.28±0.71, p=0.75).
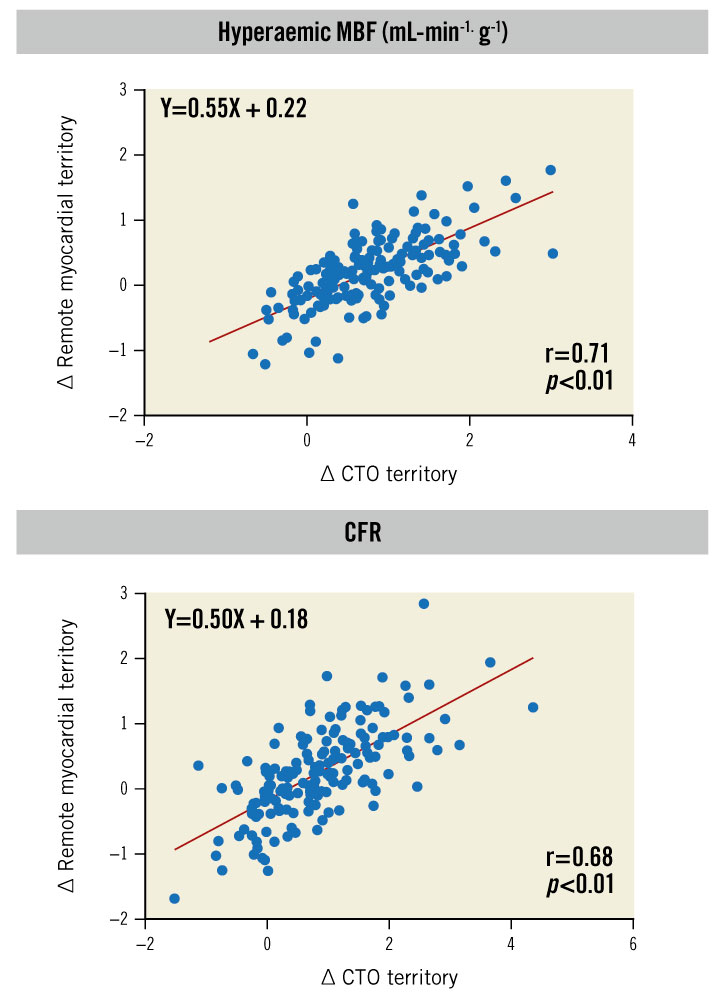
Figure 2. Association between ∆ PET perfusion CTO territory versus ∆ remote myocardial territory. Scatterplots demonstrating the correlation between the change in absolute myocardial perfusion in the CTO and the remote myocardial territory following CTO PCI. In patients with a larger increase in hMBF and CFR in CTO myocardium following CTO PCI, a larger increase in quantitative perfusion indices was observed in remote myocardium. CFR: coronary flow reserve; CTO: chronic total occlusion; hMBF: hyperaemic myocardial blood flow; MBF: myocardial blood flow; PCI: percutaneous coronary intervention; PET: positron emission tomography
Variables associated with change in remote myocardial perfusion
There was a negative correlation between baseline remote myocardial perfusion and the change in perfusion in remote myocardium after CTO PCI (hMBF: r=−0.23, p<0.01, CFR: r=−0.31, p<0.01). Furthermore, a negative correlation was observed between baseline perfusion in the myocardium subtended by the CTO and the change in remote myocardial perfusion (hMBF: r=−0.23, p<0.01, CFR: r=−0.26, p<0.01) (Supplementary Figure 2). The change in hMBF in remote myocardium was higher in patients with a large baseline perfusion defect (≥4 segments) in the CTO territory (p=0.02). Clinical (except female gender, p=0.02 for hMBF), angiographic and procedural characteristics were not predictive of absolute changes in remote myocardial perfusion (Supplementary Table 3). The analyses limited to remote myocardium non-adjacent to the CTO territory yielded similar results (Supplementary Figure 3-Supplementary Figure 5).
Discussion
The present study evaluated the impact of successful single-vessel CTO PCI on the change in absolute myocardial perfusion in the remote myocardial territory supplied by non-target coronary arteries with the following major findings. First, absolute perfusion indices in remote and CTO myocardium showed a positive linear correlation, before and after CTO revascularisation. Second, an overall increase in absolute hMBF and CFR in remote myocardium was observed following CTO PCI. Third, the quantitative improvement in hMBF and CFR in the CTO territory was independently associated with the absolute increase in remote myocardial perfusion after CTO PCI. Finally, lower baseline perfusion indices in remote and CTO myocardium were associated with the increase in absolute remote myocardial perfusion.
Physiological connection between CTO and remote myocardial territory
Coronary haemodynamics in patients with CTOs are complex since absolute perfusion in CTO myocardium largely depends on the function of the collateral circulation5. Myocardium subtended by the CTO and remote myocardium supplied by collateral donor arteries are connected by collateral channels and, as such, compose one large myocardial territory. In the present study, we found a positive association between absolute perfusion values in CTO and remote myocardium, both before and after CTO revascularisation. Several studies have demonstrated that, even despite well-developed collaterals, collateral supply is not sufficient during conditions of increased demand, leading to myocardial ischaemia in the CTO territory in over 90% of patients27. One could argue that a supply-demand mismatch is present in ischaemic myocardium subtended by the CTO, with maximally recruited collateral supply being insufficient to prevent diminished perfusion in the CTO territory, yet simultaneously resulting in reduced perfusion in remote myocardium4. In addition, the correlation between perfusion indices in remote and CTO myocardium might be largely attributable to the extent of microvascular disease. Van de Hoef et al described a paired relationship between microvascular dysfunction in significantly obstructed coronary arteries and remote non-obstructed reference vessels in the same patient, which may be part of a global cardiac phenomenon of microvascular and endothelial disease23. Furthermore, Ladwiniec et al observed abnormal CFR in 71% of patients after CTO PCI, with concomitant abnormal CFR in >50% of patients in non-obstructed remote coronary arteries24. These findings substantiate the proposed interplay between the coronary haemodynamics in the CTO and remote myocardium before and after CTO revascularisation.
Increase in remote myocardial perfusion after CTO PCI
Prior studies have shown coronary flow and absolute myocardial perfusion improvement in the CTO territory following CTO PCI4612. In addition, several reports demonstrated an instantaneous and subsequent further regression in the function of the collateral circulation after CTO revascularisation91112. Before antegrade flow in the CTO artery is restored, collateral flow to the CTO territory enlarges the myocardial mass supplied by the remote collateral donor arteries13. Leone et al investigated the influence of the amount of perfused myocardial tissue on FFR values and in this context reported a significant association between the presence of a collateralised CTO and lower FFR measurements in collateral donor arteries25. Germane to this, several small studies showed an increase in FFR and a decrease in average peak flow velocity in the collateral donor arteries after CTO PCI. This was related to a concomitant reduction in collateral flow to the CTO myocardium, even in the absence of obstructive CAD in the collateral donor arteries813. In line with these findings, the increase of FFR in collateral donor arteries after CTO PCI may result in reclassification of vessels from below to above the ischaemic thresholds14. Based on these observations, a change in absolute remote myocardial perfusion induced by collateral regression after CTO PCI might be hypothesised. Indeed, the present study demonstrates a significant increase in hMBF and CFR in remote myocardium after single-vessel CTO PCI. In contrast, Keulards et al performed thermodilution measurements in the collateral donor artery after CTO PCI (N=10) and did not find differences in stress flow26. In addition, two studies evaluated remote myocardial perfusion values after CTO PCI with cardiovascular magnetic resonance imaging using quantitative (N=17) and qualitative (N=26) perfusion assessment, and could not demonstrate perfusion increases2728. The discordant results of these small-sized reports compared to our study may be related to differences in sample size and applied perfusion imaging methods. The significant increase in remote myocardial perfusion following CTO revascularisation may have several explanations. First, the capacity of the collateral donor arteries before CTO PCI is adapted to supply both CTO and remote myocardium. After the restoration of antegrade flow in the CTO vessel and subsequent collateral regression over time, flow velocity decreases and coronary perfusion pressure may significantly increase in the vascular territory of the collateral donor arteries, leading to enhanced perfusion in remote myocardium. Second, CTO PCI is associated with an increase in left ventricular performance which may improve overall hyperaemic perfusion conditions, including those in remote myocardium1029. Third, in patients with stable obstructive lesions causing myocardial ischaemia, Uren et al demonstrated reduced coronary vasodilator reserve in remote myocardium subtended by non-obstructed coronary arteries, which may be attributed to increased work load and the contractile response of the remote regions to compensate for temporary dysfunction in the ischaemic myocardium30. In concordance, we found a positive correlation between the absolute myocardial perfusion increase in the CTO territory and improvement in remote myocardial perfusion following CTO PCI. Successful CTO revascularisation with potential adaptation of global microvascular autoregulation results in collinear improvement in both CTO and remote myocardial perfusion.
Limitations
First, this is a single-centre experience. Second, according to contemporary CTO expert consensus, a CTO is defined as a luminal occlusion of a coronary artery with a documented or estimated duration of ≥3 months without antegrade flow through the lesion (TIMI 0)18. However, the present study recruited patients with TIMI flow grade 0-1 in the CTO vessel as the study enrolment period largely preceded the establishment of the current CTO definition which mandates a TIMI 0 lesion. Although the criteria to define a CTO lesion as applied in our study deviate from the contemporary CTO definition, the large proportion of well-developed collaterals observed in the included patient population suggest that our cohort is suitable to explore the impact of PCI CTO on changes in absolute perfusion in remote myocardium. Third, invasive pressure measurements, flow velocity assessment and collateral function evaluation have not been performed in the CTO and collateral donor arteries; hence physiological alterations in pressure and flow could not be related to changes in absolute myocardial perfusion following CTO PCI. Fourth, although patients were prospectively recruited, only subjects with serial [15O]H2O PET perfusion imaging available were included.
Conclusions
CTO revascularisation resulted in a significant increase in absolute remote myocardial perfusion. Furthermore, the quantitative improvement in stress flow and coronary flow reserve in the CTO territory was independently associated with the absolute perfusion increase in remote myocardial regions. As such, CTO PCI may have a favourable physiologic impact beyond the intended treated myocardium.
Impact on daily practice
The presence of a concomitant CTO results in alterations in coronary haemodynamics in remote myocardium subtended by the collateral donor coronary arteries. In the present study, we found that CTO PCI resulted in a significant increase in quantitative myocardial perfusion in remote myocardium, which was independently associated with the perfusion increase in the CTO territory. The beneficial effect of successful CTO PCI on absolute myocardial perfusion may extend beyond the revascularised CTO territory.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

