Abstract
Background: Randomised studies of percutaneous coronary intervention (PCI) in patients with chronic total occlusion (CTO) have shown inconsistent outcomes, suggesting incomplete understanding of this cohort and their coronary physiology. To address this shortcoming, we designed a prospective observational study to measure the recovery of absolute coronary blood flow following successful CTO PCI
Aims: We sought to identify patient and procedural characteristics associated with a favourable physiological outcome after CTO PCI.
Methods: Consecutive patients with a CTO subtending viable myocardium underwent PCI utilising contemporary techniques and the hybrid algorithm. Immediately after PCI, and at 3-month follow-up, physiological measurements were performed utilising continuous thermodilution.
Results: A total of 81 patients were included with a mean age of 63.6±8.9 years, and 66 (81.5%) were male. Physiological measurements of absolute coronary blood flow in the CTO vessel increased by 30% (p<0.001) and microvascular resistance reduced by 16% (p<0.001) from immediately post-CTO PCI to follow-up assessment. Fractional flow reserve increased by 0.02 (p=0.015) in the same period. Prior coronary artery bypass graft (CABG) and a higher estimated glomerular filtration rate (eGFR) were associated with a larger change in absolute flow. An extraplaque strategy was associated with a smaller change in absolute flow.
Conclusions: Post-CTO PCI, there is a continued augmentation in absolute coronary blood flow and reduction in microvascular resistance from baseline to follow-up at 3 months. Prior CABG and a higher baseline eGFR were predictors of a larger change in absolute coronary flow, whilst an extraplaque final wire path strategy predicted a smaller change. Lastly, the patient characteristics and comorbidities had a larger influence than procedural factors on the observed change in absolute flow.
Introduction
Percutaneous coronary intervention (PCI) in chronic coronary syndromes (CCS) is considered to improve symptoms by increasing coronary blood flow following the return of vessel patency1. Chronic total occlusions (CTO) can be considered a special subset of coronary lesions within the spectrum of CCS. Firstly, CTO vessels have a higher atherosclerotic burden of disease and a higher incidence of severe calcification2. Secondly, CTO PCI carries a higher risk of complication, including periprocedural infarction and major branch occlusion3. Finally, vessels that contain a CTO have a considerably greater collateral supply compared with vessels affected by subocclusive disease4. Therefore, CTO PCI represents a complex intervention that could possibly have different outcomes to PCI for subocclusive disease and, thus, requires exclusive scientific studies to assess its efficacy. Randomised studies looking at outcomes following PCI have revealed inconsistent results5678, suggesting that a more in-depth understanding of CTO PCI patients and their coronary physiology is required. There is a need for a robust, objective method for determining procedural success beyond angiography, and coronary physiology allows us to document a quantitative change following CTO PCI. In the absence of direct measurements of flow, this has been demonstrated by different invasive physiological measurements considered surrogates of flow, such as Doppler-derived coronary flow reserve (CFR)9, fractional flow reserve (FFR)10 and non-invasive methods like myocardial positron emission tomography (PET)11. All these modalities have drawbacks, including indirect estimation of flow (e.g., FFR), a lack of operator independence for invasive measurements and the absence of vessel-specific assessment for non-invasive methods.
Therefore, there remains an opportunity to utilise more novel techniques, specifically continuous thermodilution, which has the benefit of being both operator-independent and vessel-specific12.
The aim of this study was to use continuous thermodilution to document the change in coronary flow following successful CTO PCI. Furthermore, we sought to identify both patient- and procedural-related predictors of the change in flow.
Methods
Study design & population
This was a prospective, multicentre, observational study of culprit vessel physiology following successful CTO PCI (ClinicalTrials.gov: NCT03830853). Patients scheduled for elective, clinically indicated CTO PCI with demonstrable viability of myocardium in the CTO-subtended territory were recruited (inclusion and exclusion criteria are detailed in Supplementary Appendix 1). The participating centres were the Essex Cardiothoracic Centre (Basildon, UK), Royal Sussex County Hospital (Brighton, UK) and Catharina Hospital (Eindhoven, the Netherlands). Invasive physiological measurements were performed immediately following successful CTO PCI (index) and repeated at 3 months after the index procedure (90-day follow-up).
CTO-subtended myocardium was considered viable if there was normal left ventricular function, as assessed by either transthoracic echocardiography, left ventricular angiography or cardiac magnetic resonance imaging (cMRI). When assessed by cMRI, late gadolinium enhancement of less than 50% of myocardial wall thickness was defined as being viable.
All patients provided written informed consent, and the study had local regional ethical committee approval. The study adhered to the principles of the Declaration of Helsinki.
Chronic total occlusion percutaneous coronary intervention
CTO PCI was performed according to contemporary techniques utilising the hybrid algorithm. Prior to the procedure, patients were commenced on dual antiplatelet therapy. Recanalisation strategy was at the operator’s discretion. The use of intravascular ultrasound (IVUS) was strongly encouraged. A procedure was considered technically successful when achieving Thrombolysis in Myocardial Infarction (TIMI) flow grade 2 or greater with <30% angiographic residual stenosis in the CTO vessel by visual assessment13. All patients received drug-eluting stents. Patients were discharged on dual antiplatelet therapy for a minimum of 6 months.
Physiological measurements
After successful CTO PCI, coronary physiology measurements were performed using the continuous thermodilution method. For this purpose, a pressure/thermistor guidewire (Pressure Wire X; Abbott Vascular), a monorail infusion catheter (RayFlow; Hexacath) and a dedicated software system (CoroFlow v3.01; Coroventis) were used. Absolute coronary blood flow was measured using the previously published methodology14. In brief, the dedicated infusion catheter was positioned in the proximal vessel and the pressure wire, after equalisation of pressure and temperature, was placed in the distal vessel at least 60 mm from the tip of the infusion catheter. This pressure wire position was documented at the index procedure with an angiographic acquisition; this image was reviewed at follow-up to ensure that the pressure wire was placed at the same location. Room temperature saline was infused (20-25 ml/min for the left anterior descending artery [LAD] and left circumflex artery [LCx]; 15-20 ml/min for the right coronary artery [RCA]) and the reduction in mixed blood temperature (T) was measured. The saline infusion itself creates a hyperaemic state, and additional adenosine is not necessary1516. A steady state was reached within 10-15 seconds and the pressure wire was pulled back into the infusion catheter to determine the infusion temperature (Ti). Absolute flow (Q, ml/min) was calculated utilising the Coroventis software (CoroFlow v3.01) alongside microvascular resistance (Rmicro, Wood units [WU]) and continuous thermodilution-derived fractional flow reserve (FFR).
Follow-up
Patients with a completed physiological assessment at the time of the CTO PCI procedure were scheduled for follow-up at 3 months. Patients underwent repeated physiological assessments as per the index procedure. Any optimisation of CTO PCI or further treatment was undertaken only after completion of research measurements.
Statistical analysis
Continuous data are expressed as mean (standard deviation) or median (25th and 75th percentile) depending on the distribution of data, and categorical data are expressed as percentages. Normality of data distribution was assessed by the Shapiro-Wilk test. Comparisons of index and follow-up procedure data were performed with a paired t-test or the Wilcoxon signed-rank test. Two independent samples were compared with an independent t-test or the Mann-Whitney U test, as appropriate. Simple linear regression models were used to evaluate the associations between each preselected clinically significant parameter and change in absolute coronary flow from index to follow-up. Within a hierarchical multivariate model, specific patient characteristics associated with coronary artery disease (age, diabetes, hypertension, and hypercholesterolaemia) or increased complexity of procedure (prior coronary artery bypass graft [CABG]) were included within the base model and potentially modifiable procedural factors (extraplaque final wire path, length of stent and maximal balloon diameter) were added into the multiple regression analysis including variables with a p-value <0.10 on univariate analysis. Procedural variables that demonstrated collinearity were not included together. Model assumptions were checked, such as multicollinearity. A two-tailed p-value of <0.05 was considered significant. The statistical analyses were performed using SPSS 26 (IBM).
Results
Study population
The study flowchart is included in Supplementary Figure 1. An initial 119 patients underwent a CTO PCI procedure, of which 106 (89%) had a successful procedure. In total, 81 patients completed physiological assessments utilising continuous thermodilution of the CTO vessel immediately after PCI and at follow-up. The median interval between the index procedure and follow-up was 77 (interquartile range [IQR] 61-97) days. The timing of some follow-up physiological measurements were expedited due to the impact of the COVID-19 pandemic.
Patient characteristics
Full patient characteristics are displayed in Table 1. The mean age was 64±9 years, and 66 (81.5%) were male. Sixty-three percent of patients had CCS angina class II or higher. Details of medical therapy are displayed in Supplementary Table 1. Of the 44 (54%) patients who underwent ischaemia testing, 41 (93%) had an ischaemic burden ≥10%, and the overall mean ischaemic burden of the CTO territory was 19.0±8.4%. The median left ventricular ejection fraction was 55% (IQR 48-60%).
Table 1. Baseline characteristics.
| Mean±SD/N (%) | |||
|---|---|---|---|
| Demographics | Age | 63.6±8.9 | |
| Male | 66 (81.5) | ||
| BMI, kg/m2 | 29.2±4.4 | ||
| eGFR, ml/min | 88.6±25.0 | ||
| Cardiovascular risk factors | Hypertension | 57 (70.4) | |
| Current smoker | 12 (14.8) | ||
| Diabetes mellitus | 16 (19.8) | ||
| Dyslipidaemia | 71 (87.7) | ||
| Previous myocardial infarction | 41 (50.6) | ||
| Prior CABG | 12 (14.8) | ||
| Symptoms | CCS angina class | CCS I | 18 (22.2) |
| CCS II | 37 (45.7) | ||
| CCS III | 23 (28.4) | ||
| CCS IV | 3 (3.7) | ||
| NYHA Class (I/II/III/IV) | NYHA I | 35 (43.2) | |
| NYHA II | 27 (33.3) | ||
| NYHA III | 19 (23.5) | ||
| NYHA IV | 0 (0) | ||
| Medication | No. of antianginal agents | 2 (IQR 1-3) | |
| Viability assessment | LVEF, % | 55±11x² | |
| Viability if LVEF <50% (n=23) | MIBI | 3 (13.0) | |
| MRI | 17 (73.9) | ||
| Stress echo | 3 (13.0) | ||
| Ischaemia assessment (n=44) | Ischaemic burden, % | 19±8.4 | |
| Ischaemia ≥10% | 41 (93.2) | ||
| BMI: body mass index; CABG: coronary artery bypass graft; CCS: coronary calcium score; eGFR: estimated glomerular filtration rate; IQR: interquartile range; LVEF: left ventricular ejection fraction; MIBI: myocardial perfusion imaging; MRI: magnetic resonance imaging; NYHA: New York Heart Association; SD: standard deviation | |||
Anatomical characteristics
The anatomical characteristics are described in Table 2. The CTO vessel frequency was as follows: RCA: 65%; LAD: 25%; and LCx: 10%. The proportion of patients with a Japanese CTO (J-CTO) score ≥2 was 63%.
Table 2. Anatomical characteristics.
| Mean±SD/median (IQR)/N (%) | |||
|---|---|---|---|
| CTO vessel | Right coronary artery | 53 (65) | |
| Left anterior descending artery | 20 (25) | ||
| Circumflex artery | 8 (10) | ||
| Major donor vessel | Right coronary artery | 20 (25) | |
| Left anterior descending artery | 50 (62) | ||
| Circumflex artery | 8 (10) | ||
| SVG | 2 (3) | ||
| LMS | 1 (1) | ||
| Lesion characteristics | Tapered/blunt | 35 (43) | |
| Calcification present | 46 (57) | ||
| Bending >45 degrees | 28 (35) | ||
| Length >20 mm | 44 (54) | ||
| Previous attempt | 13 (16) | ||
| Total J-CTO score | J-CTO 0 | 12 (15) | |
| J-CTO 1 | 18 (22) | ||
| J-CTO 2 | 20 (25) | ||
| J-CTO 3 | 21 (26) | ||
| J-CTO 4 | 8 (10) | ||
| J-CTO 5 | 2 (2) | ||
| Collateral filling | None | 1 (1) | |
| Bridge collaterals | 6 (8) | ||
| Retrograde filling | 40 (50) | ||
| Both | 33 (41) | ||
| Collateral size | CC0 | 4 (5) | |
| CC1 | 43 (54) | ||
| CC2 | 20 (25) | ||
| CC3 | 13 (16) | ||
| Rentrop grade | 0 | 0 (0) | |
| 1 | 3 (4) | ||
| 2 | 25 (31) | ||
| 3 | 52 (65) | ||
| CTO: chronic total occlusion; J-CTO: Japanese CTO score; IQR: interquartile range; LMS: left main stem; SD: standard deviation; SVG: saphenous vein graft | |||
Procedural characteristics
The procedural characteristics are reported in Table 3, with detailed procedural observations in Supplementary Table 2. The successful crossing strategies are summarised as follows: anterior wire escalation (AW): 64.2%; retrograde wire escalation (RW): 2.5%; antegrade dissection re-entry (ADR): 8.6%; and retrograde dissection re-entry (RDR): 24.7%. The mean number of stents was 2±2 stents, and the mean length of stented segments was 68.4±30.3 mm. The maximum stent and balloon diameters were 3.5 (IQR 3.0-3.5) mm and 3.5 (IQR 3.0-4.0) mm, respectively. Post-procedural TIMI 3 flow was achieved in 79 (98%) patients, and the remaining 2/81 (2%) had TIMI 2 flow.
Table 3. Procedural characteristics.
| Mean±SD/median (IQR)/N (%) | ||
|---|---|---|
| Successful crossing strategy | AW | 52 (64) |
| RW | 2 (3) | |
| ADR | 7 (9) | |
| RDR | 20 (25) | |
| Lumen re-entry technique if dissection | CART | 0 (0.0) |
| Reverse CART | 17 (21.0) | |
| Stingray | 3 (3.7) | |
| LAST | 6 (7.4) | |
| STAR | 1 (1.2) | |
| PCI detail | Length of stented segment | 68.4±30.3 |
| Number of stents | 2±1 | |
| I | 24 (29.6) | |
| II | 33 (40.7) | |
| II | 19 (23.5) | |
| IV | 5 (6.2) | |
| Max stent diameter | 3.5±0.5 | |
| Max balloon diameter | 3.5±1.0 | |
| Predicted subintimal length (n=26) | 22.65±12.20 | |
| Duration and radiation | Procedure time (min) | 171±56.5 |
| Wire time (min) | 38±59 | |
| Contrast volume (ml) | 282±89 | |
| DAP (cGycm2) | 20,013±12,580 | |
| Skin dose (mGy) | 2,804±1,353 | |
| ADR: antegrade dissection re-entry; AW: antegrade wire escalation; CART: controlled antegrade and retrograde tracking and dissection; DAP: dose area product; IQR: interquartile range; LAST: limited antegrade subintimal tracking; PCI: percutaneous coronary intervention; RDR: retrograde dissection re-entry; RW: retrograde wire escalation; SD: standard deviation | ||
Changes in absolute flow (Q)
The median absolute coronary blood flow in the CTO vessel at index and follow-up were 149 (IQR 121-205) ml/min and 201 (IQR 155-205) ml/min, respectively, delta +30% (IQR 6-51); p<0.001 (Central illustration). On a per vessel analysis, the median change in absolute flow was as follows: RCA 47.0 (IQR 12.0-90.0) ml/min; p<0.001; LAD 45.5 (IQR 11.0-58.5) ml/min; p<0.001; and LCx 39.5 (3.8-92.5) ml/min; p=0.152 (Figure 1).
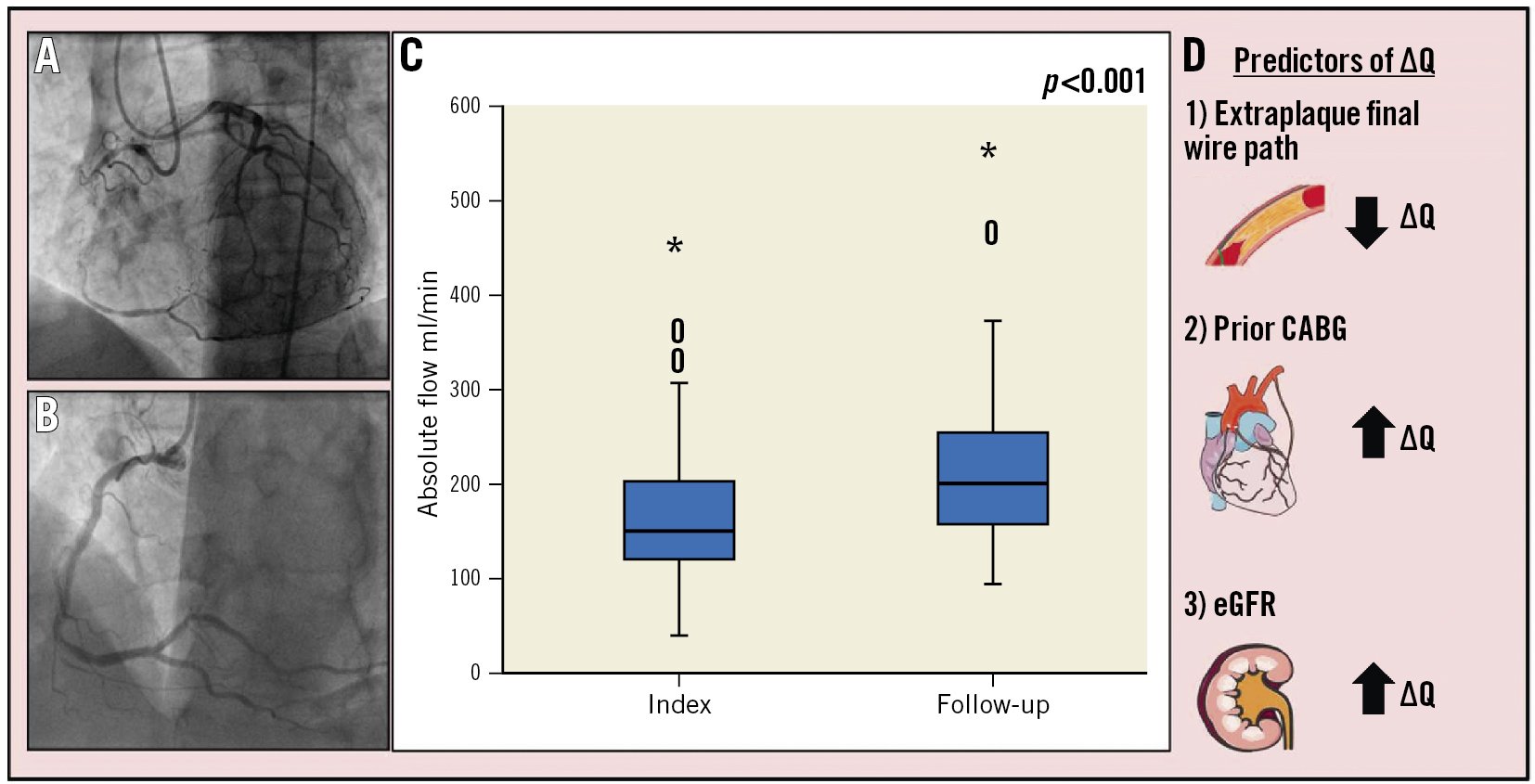
Central illustration. Absolute coronary flow after successful CTO PCI and clinical predictors of change in flow. Patients underwent CTO-vessel PCI (A) followed by immediate measurements of absolute flow in the culprit vessel. These measurements were repeated at follow-up (B). Absolute coronary flow increased from index to follow-up by 30% (C). Predictors of change in absolute flow (ΔQ) (D); an extraplaque final wire path predicted a smaller change in absolute flow, whereas prior CABG and a higher eGFR predicted a larger change in absolute flow. CABG: coronary artery bypass graft; CTO: chronic total occlusion; eGFR: estimated glomerular filtration rate; PCI: percutaneous coronary intervention
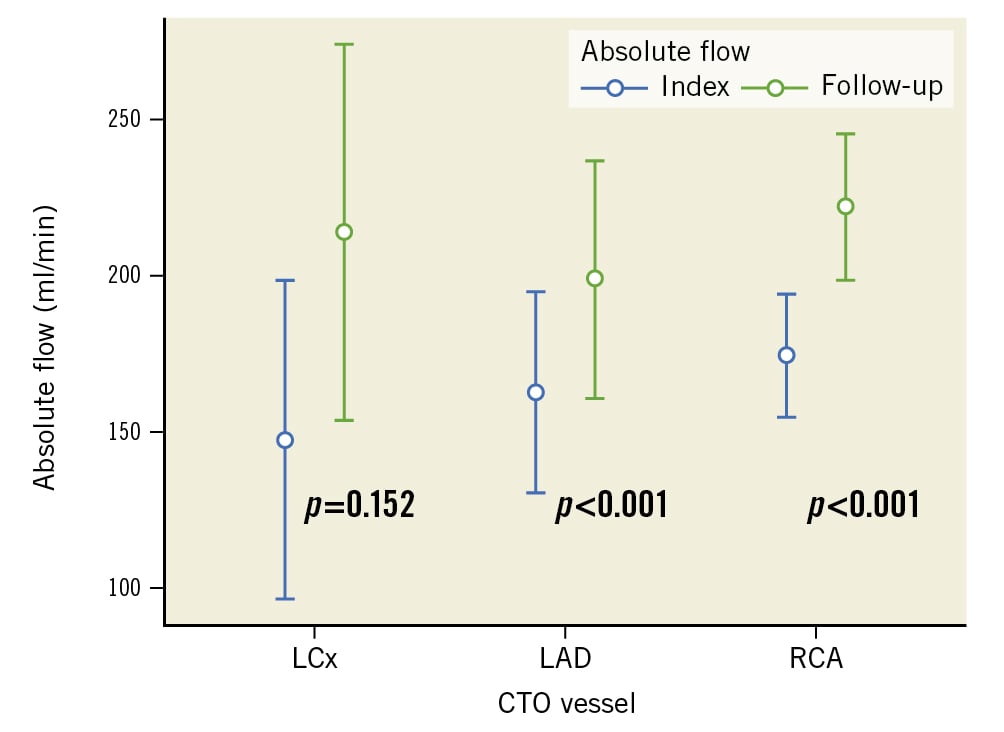
Figure 1. Absolute coronary blood flow at index and follow-up per vessel. Absolute coronary blood flow at index and follow-up by CTO vessel. Bars: interquartile range (IQR). Dots: median value. CTO: chronic total occlusion; LAD: left anterior descending artery (n=20); LCx: left circumflex artery (n=8); RCA: right coronary artery (n=53)
Changes in resistance
The median microvascular resistance (Rmicro) at index and follow-up were 453 (IQR 353-579) WU and 370 (IQR 286-471) WU, respectively, delta −70 (IQR −168 to −2) WU; p<0.001. The change in epicardial (Repi) and total vessel resistance (Rtot) was −30 (IQR −83 to 5) WU and −115 (IQR −254 to −35) WU, respectively; p<0.001 for both (Table 4). The Spearman’s rank correlation (rho) for the correlation of change in Rmicro and change in Repi to change in absolute coronary flow were 0.522 (p<0.001) and 0.322 (p=0.322), respectively (Supplementary Table 3). Detailed histograms of absolute flow and resistance variables are presented in Supplementary Figure 2 and Supplementary Figure 3.
Table 4. Resistance measurements at index immediately after CTO PCI and at 3-month follow-up by vessel.
| Index (median [IQR]) WU | Follow-up (median [IQR]) WU | Delta (median [IQR]) WU[% (IQR)] | p-value | |
|---|---|---|---|---|
| Total resistance (Rtot) | 569 (450-703) | 433 (333-550) | −254 (−115 to −35)[−24% (−37 to −6)] | <0.001 |
| RCA | 559 (449-674) | 432 (333-515) | −113 (−253 to −35) | <0.001 |
| LAD | 617 (465-823) | 462 (367-606) | −120 (−243 to −42) | <0.001 |
| LCx | 674 (451-905) | 421 (335-602) | −212 (−358 to −54) | 0.078 |
| Microvascular resistance (Rmicro) | 453 (353-579) | 370 (286-471) | −70 (−168 to −2)[−16% (−33 to −0.4)] | <0.001 |
| RCA | 468 (356-555) | 367 (288-452) | −72 (−168 to −2) | <0.001 |
| LAD | 424 (332-581) | 373 (272-482) | −52 (−149 to −9) | 0.004 |
| LCx | 540 (360-671) | 388 (282-532) | −66 (−174 to −11) | 0.250 |
| Epicardial resistance (Repi) | 101 (37-162) | 60 (33-94) | −30 (−83 to 5)[−36% (−64 to −6)] | <0.001 |
| RCA | 51 (31-145) | 47 (31-82) | −19 (−64 to 13) | 0.003 |
| LAD | 137 (114-204) | 95 (64-123) | −52 (−83 to −32) | <0.001 |
| LCx | 79 (18-157) | 54 (40-72) | −10 (−132 to 38) | 0.547 |
| Rtot = Pa/Q; Rmicro =Pd/Q; Repi= (Pa-Pd)/Q. CTO: chronic total occlusion; IQR: interquartile range; LCx: circumflex artery; LAD: left anterior descending artery; Pa: aortic pressure; PCI: percutaneous coronary intervention; Pd: distal coronary pressure; Q: absolute coronary flow; RCA: right coronary artery; WU: Wood units | ||||
Changes in fractional flow reserve (FFR)
The median FFR at index and follow-up were 0.84 (IQR 0.73-0.93) and 0.86 (IQR 0.79-0.91), respectively, delta +0.02 (IQR −0.04 to 0.10); p=0.015. On a per vessel analysis, the median change in FFR was as follows: RCA 0.02 (IQR −0.05 to 0.07); p=0.138; LAD 0.04 (IQR −0.003 to 0.11); p=0.017; LCx −0.005 (IQR −0.10 to 0.17); p=0.554 (Table 5).
Table 5. FFR at index and follow-up.
| Index(median [IQR]) | Follow-up(median [IQR]) | Delta(median [IQR]) | p-value | |
|---|---|---|---|---|
| FFR | 0.84 (0.73-0.93) | 0.86 (0.79-0.91) | 0.02 (−0.04 to 0.10) | 0.015 |
| RCA | 0.87 (0.75-0.95) | 0.89 (0.81-0.93) | 0.02 (−0.05 to 0.07) | 0.138 |
| LAD | 0.76 (0.68-0.82) | 0.80 (0.74-0.85) | 0.04 (−0.003 to 0.11) | 0.017 |
| LCx | 0.86 (0.68-0.98) | 0.88 (0.84-0.89) | −0.005 (−0.10 to 0.17) | 0.554 |
| IQR: interquartile range; FFR: fractional flow reserve; LCx: circumflex artery; LAD: left anterior descending artery; RCA: right coronary artery | ||||
Patient characteristics as predictors of change in absolute coronary flow
Univariate linear regression analysis of patient characteristics identified age (unstandardised correlation coefficient B= −1.93, 95% confidence interval [CI]: −3.38 to −0.48; p=0.01), diabetes (B= −43.53, 95% CI: −75.72 to −11.34; p=0.009) and estimated glomerular filtration rate (eGFR; B=0.61, 95% CI: 0.09 to 1.13; p=0.021) as predictors of change in absolute coronary flow (Table 6). Increased age and diabetes were associated with a smaller change in absolute flow, whereas a higher eGFR was associated with a larger change.
Table 6. Univariate and multiple linear regression on change in absolute flow immediately post CTO PCI and follow-up.
| Simple linear regression model | Multiple regression model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | t value | p-value | Beta | B (95% CI) | t value | p-value | Beta | ||
| Patient characteristics | Age | −1.93(−3.38 to −0.48) | −2.655 | 0.01 | −0.286 | −1.41 (−2.83 to 0.01) |
−1.974 | 0.052 | −0.208 |
| Male gender | 17.29 (−16.97 to 51.55) |
1.005 | 0.317 | 0.112 | |||||
| BMI | 0.62 (−2.43 to 3.68) |
0.406 | 0.686 | 0.046 | |||||
| Diabetes | −43.53 (−75.72 to −11.34) | −2.691 | 0.009 | −0.290 | −20.09 (−54.02 to 13.84) |
−1.181 | 0.242 | −0.134 | |
| HTN | 0.32 (-29.01,– 29.65) | 0.021 | 0.983 | 0.002 | 1.10 (−25.43 to 27.63) |
0.083 | 0.934 | 0.008 | |
| Hyper-cholesterolaemia | −1.65 (−42.36 to 39.06) | −0.081 | 0.936 | −0.009 | −7.16 (−45.42 to 31.11) |
−0.373 | 0.710 | −0.039 | |
| Smoker | 1.5 (−36.20 to 39.20) | 0.079 | 0.937 | 0.009 | |||||
| Prior MI | −13.13 (−39.75 to 13.50) | −0.981 | 0.329 | −0.110 | |||||
| Previous PCI | 12.77 (−14.34 to 39.87) |
0.938 | 0.351 | 0.105 | |||||
| Prior CABG | 30.261 (−6.82 to 67.35) | 1.624 | 0.108 | 0.180 | 41.71 (6.37 to 77.04) |
2.354 | 0.021 | 0.248 | |
| eGFR | 0.61 (0.09 to 1.13) | 2.352 | 0.021 | 0.256 | 0.62 (0.09 to 1.16) |
2.322 | 0.023 | 0.262 | |
| LVEF | 0.08 (−1.28 to 1.43) | 0.112 | 0.911 | 0.013 | |||||
| Ischaemic burden | 0.08 (−2.29 to 2.46) | 0.072 | 0.943 | 0.012 | |||||
| Anatomical characteristics | J-CTO score >2 (ref: J-CTO ≤2) | −8.32(−35.81 to 19.17) | −0.602 | 0.549 | −0.068 | ||||
| In-stent CTO | 5.52 (−45.602 to 56.642) | 0.215 | 0.83 | 0.024 | |||||
| Collateral flow | Ipsilateral | −98.208 (−228.226 to 31.809) | −1.504 | 0.137 | −0.433 | ||||
| Contralateral | −89.010 (−210.878 to 32.858) | −1.455 | 0.15 | −0.744 | |||||
| Both | −86.747 (−208.93 to 35.436) | −1.414 | 0.161 | −0.714 | |||||
| CC grade ≥2- ref Grade <2 | 16.81 (−10.34 to 43.96) | 1.233 | 0.221 | 0.138 | |||||
| Rentrop grade ≥2 - ref grade <2 | −22.12 (−87.74 to 43.50) | −0.671 | 0.504 | −0.076 | |||||
| End-TIMI grade III (ref: TIMI 2) | −102.475 (−185.668 to −19.281) | −2.452 | 0.016 | −0.266 | |||||
| Post-PCI Rentrop grade- ref 0 | Grade 1 | 4.923 (−27.291 to 37.136) | 0.306 | 0.761 | 0.043 | ||||
| Grade 2 | 4.381 (−39.993 to 48.750) | 0.198 | 0.844 | 0.028 | |||||
| Procedural characteristics | Extraplaque* final wire path | −31.056 (−58.600 to −3.510) | −2.244 | 0.028 | −0.245 | −39.61 (−72.98 to −6.24) |
−2.367 | 0.021 | −0.312 |
| Crossing strategy (ref RDR) * | AW | 30.304 (−0.809 to 61.416) | 1.939 | 0.056 | 0.243 | ||||
| RW | 50.650 (−37.043 to 138.343) | 1.150 | 0.254 | 0.131 | |||||
| ADR | 0.007 (−51.921 to 51.935) | 0.000 | 1.00 | 0.000 | |||||
| Pred extraplaque length* | −0.56 (−1.69 to −0.57) | −0.992 | 0.324 | −0.113 | |||||
| Method of lumen entry (ref: none)* | Reverse CART | −35.672 (−68.749 to −2.595) | −2.148 | 0.035 | −0.243 | ||||
| Stingray | −18.574 (−89.125 to 51.977) | −0.524 | 0.602 | −0.059 | |||||
| LAST | −22.907 (−74.090 to 28.275) | −0.891 | 0.376 | −0.100 | |||||
| STAR | −38.907 (−158.942 to 81.127) | −0.646 | 0.521 | −0.072 | |||||
| Max balloon diameter | 8.316 (−19.890 to 36.521) | 0.587 | 0.559 | 0.066 | 2.83 (−23.28 to 28.93) |
0.216 | 0.830 | 0.022 | |
| Max stent diameter | −3.596 (−32.420 to 25.228) | −0.248 | 0.805 | −0.028 | |||||
| Total length of stents | −0.054 (−0.499 to 0.39) | −0.242 | 0.809 | −0.027 | 0.16 (−0.31 to 0.62) |
0.677 | 0.501 | 0.080 | |
| Number of stents | −3.091 (−18.298 to 12.116) |
−0.405 | 0.687 | −0.045 | |||||
| *significant collinearity therefore not included in multiple regression together. B: unstandardised regression coefficient; Beta: standardised regression coefficient. ADR: antegrade dissection re-entry; AW: antegrade wire escalation; BMI: body mass index; CABG: coronary artery bypass graft; CART: controlled antegrade and retrograde tracking and dissection; CC: Calgary Collateral score; CI: confidence interval; CTO: chronic total occlusion; eGFR: estimated glomerular filtration rate; HTN: hypertension; J-CTO; Japanese CTO score; LAST: limited antegrade subintimal tracking; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coroanry intervention; RDR: retrograde dissection re-entry RW: retrograde wire escalation; STAR: subintimal tracking and re-entry; TIMI: Thrombolysis in Myocardial Infarction | |||||||||
Anatomical characteristics as predictors of change in absolute coronary flow
There were no clinically significant anatomical factors that were associated with change in absolute coronary flow. Specifically, neither the complexity of the lesion (J-CTO score >2), nor an in-stent occlusion location, nor the type or grade of collateral flow correlated with change in absolute flow. TIMI 3 flow in the CTO vessel post-CTO PCI was associated with a lower change in absolute flow than TIMI 2 (p-value 0.016). Further univariate analysis of the J-CTO score elements is provided in Supplementary Table 4.
Procedural characteristics as predictors of change in absolute coronary flow (ΔQ)
Univariate regression analysis for procedural factors demonstrated that only an extraplaque final wire path was a significant predictor of change in absolute flow (Table 6). When analysed by crossing strategy, compared with RDR, AW (B=30.30, 95% CI: −0.81 to 61.42; p=0.056) showed a trend towards a greater change in absolute flow than RW (B=50.65, 95% CI: −37.94 to 138.34; p=0.254) and ADR (B=0.01, 95% CI: −51.92 to 51.94; p=1.00); however, this trend did not cross the threshold for significance. The maximum diameter of the balloon or stent used, as well as the length of the stented segment, were not significantly associated with change in absolute flow (p=0.559, 0.805 and 0.809, respectively).
Table 6. Univariate and multiple linear regression on change in absolute flow immediately post CTO PCI and follow-up.
Combined patient and procedural characteristics as predictors of change in absolute flow
The results generated from the hierarchical multiple regression analysis of selected procedural characteristics on a baseline model of clinically important patient characteristics indicated that prior CABG (B=41.71, 95% CI: 6.37-77.04; p=0.021), eGFR (B=0.62, 95% CI: 0.09-1.16; p=0.023) and an extraplaque final wire path (B= −39.61, 95% CI: −72.98 to −6.24; p=0.021) were predictors of change in absolute flow (Table 6). Patient characteristics of age and diabetes, identified on univariate analysis, were no longer significant using this model. TIMI flow at the end of the procedure was not included in the multivariate analysis, as only 2 patients had TIMI grade 2 flow and the rest had grade 3. This combined model of patient and procedural characteristics explained 28.6% (R2 0.286) of variation in change in absolute flow, where the selected patient characteristics independently explained 28% and procedural factors 0.6%. An alternative multivariate model using significant univariate variables only is shown in Supplementary Table 5.
Discussion
This study provides the largest contemporary cohort of CTO patients undergoing serial invasive physiological measurements of absolute coronary flow immediately post-CTO PCI and at short-term follow-up. The three key findings were as follows: firstly, absolute coronary blood flow continued to augment by ~30% from immediately post-CTO PCI to follow-up at 3 months. Secondly, prior CABG and a higher eGFR were predictors of a larger change in absolute coronary flow. Conversely, an extraplaque final wire path strategy predicted a smaller change in absolute flow. Lastly, patient characteristics and comorbidities had a larger influence than procedural factors on the change in absolute flow.
The augmentation of coronary blood flow in the target vessel following successful CTO PCI
The understanding of post-CTO physiology has significantly improved over the previous two decades. This study demonstrates that absolute coronary flow continues to improve after CTO PCI. Similar changes of flow in the CTO vessel post-PCI have been demonstrated in CFR by Doppler9, FFR10 and PET11.
Karamasis et al10 demonstrated FFR in right coronary arteries improved immediately post-CTO PCI to follow-up (delta +0.07±0.08; p<0.001)). Similarly, within this cohort, FFR improved from index to follow-up (delta +0.02 [IQR −0.04 to 0.10]; p=0.015). This increment was demonstrated in all three epicardial vessels, although it was only statistically significant in the LAD (p=0.017). The different technique in this study, continuous thermodilution of saline-derived hyperaemia compared to adenosine, to measure FFR may reflect the difference in magnitude observed.
Myocardial blood flow measured using [15O]H2O PET perfusion imaging is considered by some as the gold standard measure of coronary blood flow, as it takes into account myocardial mass. However, absolute coronary flow using continuous thermodilution provides a highly robust and reproducible measure that is vessel specific15. This is possible as the saline infusion creates localised hyperaemia in the myocardial bed of that vessel without inducing hyperaemia in others, which occurs with systemic adenosine.
The 30% increase in absolute flow was associated with a 24% reduction in Rtot, a 16% reduction in Rmicro and a 36% reduction in Repi (all p<0.001). The large reduction in epicardial resistance is likely due to vascular remodelling of the epicardial vessel after recanalisation1718. The proportionally smaller reduction in Rmicro is likely due to initial maximal microvascular vasodilatation within the CTO-subtended myocardium19 providing minimal resistance. The subsequent reduction in microvascular resistance at follow-up has also been shown by Werner et al9 to be a transient microvascular dysfunction that improves.
Factors influencing the augmentation of coronary blood flow
The change in absolute flow over time is thought to occur due to a reduction of myocardial resistance within the subtended territory and distal vessel remodelling18. Furthermore, the observed changes in absolute flow could also be as a result of changes in the endothelial function of the previously occluded vessel2021. Our study has highlighted that there is a significant difference in the change in flow achieved between an intraplaque and extraplaque wire path. Extraplaque wiring predicted a smaller change in absolute flow. When analysed by each crossing strategy there was no significant difference; however, an AW strategy compared to RDR was associated with a larger change in absolute flow (p=0.056). A larger sample may have had sufficient power to demonstrate the difference between extra- and intraplaque strategies.
Our findings are contrary to previous literature, where no difference was identified between intraplaque or extraplaque strategies. Recovery of hyperaemic (adenosine-derived) myocardial blood flow (MBF) measured using PET was similar regardless of the crossing strategy utilised, and the crossing strategy was not predictive of hyperaemic MBF (p=0.40) on univariate analysis in 193 patients undergoing CTO PCI22. However, the authors did note significantly lower improvements in flow depending on the type of wiring utilised within the extraplaque strategy, and specifically within ADR, a subintimal tracking and re-entry (STAR) technique resulted in less favourable recovery of hyperaemic blood flow. This suggests that outcomes of extraplaque wiring are heterogenous. Extraplaque wiring causes dissections which can lead to intimal haematoma formation, endothelial dysfunction, periprocedural complications and occlusion of side branches23. Side branch loss may explain why, in our study, extraplaque wiring was associated with a lower change in absolute coronary flow, as this would lead to a lower absolute coronary flow. However, prior studies have shown no significant difference in vascular healing between crossing strategies at 12 months, although there was a non-significant increase in CTO vessel revascularisation after an extraplaque strategy24. Continuous intracoronary thermodilution provides vessel-specific data, whereas PET imaging specifically measures segmental and global myocardial blood flow, therefore, loss of side branch may influence absolute coronary blood flow measurements more than PET. Our data highlight that the procedural strategy used may impact the change in absolute flow gained, it must be remembered that an extraplaque strategy is dictated by the anatomy and complexity of the lesion, as opposed to operator choice alone, and can be associated with a higher rate of stent implantation.
The change in absolute flow is influenced by patient characteristics of eGFR, prior CABG and an extraplaque final pathway. A lower baseline eGFR may represent an increased complexity due to higher calcification of the CTO vessel2. Furthermore, patients with lower eGFR have lower coronary flow reserve compared to those with higher renal function2526. Possible coronary microvascular dysfunction (CMD), as part of a systemic process27, may be the mechanism for the lower rate of change in absolute flow; however, this requires a dedicated CMD assessment that was not performed in this study.
Of the 12 participants who had prior CABG, nine had a bypass of the target CTO vessel, and only two had an occluded graft (Supplementary Table 6). The mechanism of why prior CABG predicts a larger change in flow may be related to the continuous thermodilution technique employed to measure absolute flow. There may be a dilutional effect of the bypass graft blood interacting with the saline infusion within the recanalised CTO, providing a higher mixed blood temperature (T) and, thus, providing a lower absolute coronary flow value at index. At follow-up, the remodelled native vessel with brisker flow has a comparatively higher flow with less competitive flow from the graft and a lower mixed blood temperature and higher absolute flow. The physiological impact of bypass grafts requires further investigation as, historically, CTO PCI in this cohort is associated with a higher rate of procedural failure, likely as a consequence of multimorbidity28, and, as such, a positive signal in the change in flow has to be interpreted cautiously.
Prior literature demonstrates that the improvement of FFR, therefore a surrogate of coronary flow, leads to improved outcomes for standard (non-CTO) PCI29. Within this cohort, we are firstly demonstrating there is an improvement of absolute coronary flow with a novel method of continuous thermodilution that is operator-independent and avoids the need for adenosine-induced hyperaemia. Subsequently, future avenues of research will establish any correlation of this method to both symptomatic and prognostic outcomes in this complex cohort of chronic coronary syndrome patients.
Limitations
There are several limitations with our observational study. Our sample size is modest and, therefore, may not provide sufficient power to identify predictors with smaller but significant effect sizes. Every attempt was made to ensure the positioning of the pressure wire at follow-up was the same as at the index procedure, as described, but small deviations of the wire position cannot be excluded. However, this is the largest set of paired data utilising an operator-independent method of measuring absolute coronary blood flow which can mitigate somewhat for the limitations of size.
Determination of the extraplaque final wire path was by successful crossing strategy, therefore, dissection re-entry (ADR, RDR). There may have been instances of extraplaque wiring within wire escalation strategies (AW, RW) that were not identified at the time of the procedure. Similarly, the subintimal length was determined angiographically by the operator. The former can occur during contemporary practice of CTO PCI, whereas the latter may be mitigated with intravascular imaging. This lack of intravascular imaging data meant we could not provide detailed measurements of lesion length, minimum stent size, minimum balloon size, and minimum stent diameter or area. Finally, we could not determine the impact of vasodilatory medications at follow-up on the change in absolute flow as this was not collected and, although these agents had no significant predictive value at index (Supplementary Table 7), the impact of medications on coronary collateral flow after CTO PCI may be important.
Conclusions
This study demonstrates that absolute coronary blood flow values continue to improve over time after CTO PCI. An extraplaque crossing strategy was associated with a lower change in absolute flow and was an independent procedural predictor. However, patient characteristics contributed to a larger proportion of change in absolute flow than procedural aspects, and prior CABG and eGFR, specifically, were associated with a higher change in absolute coronary flow.
Impact on daily practice
Success of CTO PCI is judged angiographically and is performed for symptomatic benefit. We demonstrate that coronary blood flow augmentation post-CTO PCI can be objectively recorded utilising continuous thermodilution. Patient factors play a larger role than CTO recanalisation strategy on the final flow observed at 3 months. This highlights both the importance of patient selection and the strategy adopted for successful CTO PCI physiological outcomes. Future studies to correlate the change in absolute coronary flow to improvement in symptoms will be required.
Conflict of interest statement
C. Cook is a consultant for Philips, Boston Scientific, and Viz.ai; has received an institutional grant from Edwards Lifesciences; and has equity in Cerebria. N. Pijls has received institutional grants from Abbott and Hexacath; is a consultant for Abbott and GE Healthcare; and has minor equities in Philips, ASML, and HeartFlow. T. Keeble has received research grants from Boston Scientific, Volcano, Terumo, and Abbott Vascular. G. Karamasis has received honoraria from Abbott Vascular; and has received a research grant from Abbott Vascular. J. Davies has received a research grant from Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

