
Abstract
Aims: The aim of the present study was to investigate the effects of successful PCI CTO on absolute myocardial blood flow (MBF) and functional recovery.
Methods and results: Patients with a documented CTO were prospectively examined for ischaemia and viability with [15O]H2O positron emission tomography (PET) and late gadolinium enhancement cardiac magnetic resonance imaging (LGE-CMR). Sixty-nine consecutive patients, in whom PCI was successful, underwent follow-up PET and CMR after approximately 12 weeks to evaluate potential improvement of MBF as well as systolic function. After PCI, stress MBF in the CTO area increased from 1.22±0.36 to 2.40±0.90 mL·min–1·g–1 (p<0.001), whilst stress MBF in the remote area also increased significantly between baseline and follow-up PET (2.58±0.68 to 2.77±0.77 mL·min–1·g–1, p=0.01). The ratio of stress MBF between CTO and remote area was 0.49±0.13 at baseline and increased to 0.87±0.24 at follow-up (p<0.001). The MBF defect size of the CTO area decreased from 5.12±1.69 to 1.91±1.75 myocardial segments after PCI (p<0.001). Left ventricular ejection fraction (LVEF) increased significantly (46.4±11.0 vs. 47.5±11.4%, p=0.01) at follow-up.
Conclusions: The vast majority of CTO patients with documented ischaemia and viability showed significant improvement in stress MBF and a reduction of ischaemic burden after successful percutaneous revascularisation with only minimal effect on LVEF.
Abbreviations
ADR: antegrade dissection and re-entry
AWE: antegrade wire escalation
CAD: coronary artery disease
CC: collateral connection
CFR: coronary flow reserve
CMR: cardiac magnetic resonance
CTO: chronic coronary total occlusion(s)
EDV: end-diastolic volume
ESV: end-systolic volume
J-CTO: Japanese CTO score
LGE: late gadolinium enhancement
LVEF: left ventricular ejection fraction
LVF: left ventricular function
MBF: myocardial blood flow
PCI: percutaneous coronary intervention
PET: positron emission tomography
RDR: retrograde dissection and re-entry
RFR: relative flow reserve
RWE: retrograde wire escalation
SWT: segmental systolic wall thickening
Introduction
Chronic total occlusions (CTO) are observed in up to 30% of patients with known or suspected coronary artery disease (CAD). However, percutaneous coronary intervention (PCI) is attempted in only 10-30% of these patients1. The reluctance of physicians in terms of referral for PCI CTO is based on historically low success rates and the notion that patients may not benefit as collateral flow is assumed to be sufficient to prevent ischaemia or that myocardium subtended by the occluded artery is likely to be non-viable1-4. Consequently, non-invasive ischaemia and viability testing are advocated to guide revascularisation of CTO in a more judicious manner3. An ischaemic burden of 12.5% of myocardium at nuclear imaging has been identified as the optimal cut-off to select patients who will be likely to have significant ischaemia reduction after PCI CTO5. In addition, recovery of dysfunctional myocardium after PCI CTO can be anticipated in the presence of myocardial viability prior to revascularisation6. Nowadays, success rates for PCI CTO have been raised to over 90% with dual arterial access, improvement in guidewire technology, and implementation of dissection and re-entry strategies7. Without historical technical barriers for PCI CTO, focus should be directed on selecting patients most likely to benefit from percutaneous revascularisation, defined as a decrease of symptoms, improvement of left ventricular function (LVF), or significant reduction of ischaemic burden. The aim of the present study was to investigate the effects of successful PCI CTO on absolute myocardial perfusion and functional recovery.
Methods
STUDY DESIGN AND PARTICIPANTS
The study population consisted of prospectively recruited patients with a documented CTO of a native coronary artery evaluated with late gadolinium enhancement cardiac magnetic resonance imaging (LGE-CMR) and [15O]H2O positron emission tomography (PET) to detect myocardial viability and ischaemia, respectively. Patients were scheduled for LGE-CMR and PET at least twelve weeks after successful percutaneous revascularisation to evaluate the impact of the procedure on LVF and ischaemic burden. Exclusion criteria were symptomatic asthma, pregnancy, high-degree AV block, and three-vessel disease. The study was approved by the institutional Medical Ethics Review Committee and patients provided written informed consent.
ANGIOGRAPHIC CTO CHARACTERISTICS
Dual arterial coronary angiograms were evaluated by two experienced CTO operators (P. Knaapen and A. Nap). Pre-interventional angiographic CTO and collateral characteristics, procedural strategy, and procedural outcome were scored according to the following criteria. A CTO was defined as a complete occlusion with TIMI flow grade 0. Collateral pathways were identified as ipsilateral bridging, epicardial, and/or septal. The collateral connection score was graded as no visible connection (CC0), thread-like connection (CC1), or small branch-like connection (CC2)8. Collateral flow was scored based on epicardial filling of the occluded artery consistent with the Rentrop and Cohen classification, and the primary retrograde filling pattern was used to identify the dominant collateral pathway9. Angiographic CTO morphology was assessed according to the J-CTO score10. Procedural success was defined as <30% diameter stenosis and TIMI flow grade 3. Recanalisation was achieved by either antegrade wire escalation (AWE), antegrade dissection and re-entry (ADR), retrograde wire escalation (RWE), or retrograde dissection and re-entry (RDR)7.
POSITRON EMISSION TOMOGRAPHY
PET studies using [15O]H2O were performed as described previously11. Briefly, a dynamic emission scan was performed at rest, followed by an identical scan during administration of intravenous adenosine (140 µ kg–1·min–1). The ratio of stress MBF to rest MBF was defined as coronary flow reserve (CFR), and relative flow reserve (RFR) was calculated as the ratio of stress MBF of the downstream myocardial area of a CTO to stress MBF of a normal reference vascular territory (remote). A remote area was defined as a minimum of four adjacent myocardial segments supplied by a non-obstructive vessel with the highest stress MBF values. Defect size was defined as the total of segments in the downstream CTO territory with an RFR ≤0.75.
CMR IMAGING PROTOCOL
CMR was performed on a 1.5 Tesla scanner (MAGNETOM® Avanto; Siemens Healthcare, Erlangen, Germany) before and 12 weeks after PCI of CTO. Functional imaging was performed using an ECG-gated, balanced steady-state free-precession sequence in four-, three-, and two-chamber long-axis and short-axis orientations during mild expiration (typical parameters: TR/TE 3.2/1.5 ms, flip angle ~75°, voxel size 1.4×1.4×5.0 mm, temporal resolution 35-50 ms). CMR data were assessed by two experienced observers who were blinded to the time point of CMR examinations (baseline/follow-up) and clinical data. Left ventricle (LV) end-diastolic volume (EDV), LV end-systolic volume (ESV), and LV ejection fraction (LVEF) were calculated using endocardial and epicardial borders. Segmental systolic wall thickening (SWT) was defined as the percentage difference between the end-diastolic wall thickness and the end-systolic wall thickness. Dysfunctional myocardium was defined as a segment with <45% SWT. Late gadolinium enhancement images were acquired 10-15 minutes after intravenous administration of a bolus of 0.15 mmol/kg Gd-DOTA. Viable myocardium was defined as myocardium without or with ≤50% extent of hyperenhancement. Baseline ECGs of all patients were assessed for the presence of pathological Q-waves to determine the relation with CMR results.
STATISTICAL ANALYSIS
Continuous variables are presented as mean values±standard deviation (SD), whereas categorical variables are expressed as actual numbers. Continuous variables of paired data were compared with the paired sample t-test, whereas comparisons between groups were performed using the two independent samples t-test, unless stated otherwise. Paired non-normal data were analysed with the Wilcoxon signed-rank test where appropriate. Associations were evaluated using Pearson’s correlation analysis for normally distributed data or Spearman’s correlation analysis for non-normally distributed data. Differences in change of CTO MBF and LVF predicted by given variables were estimated by linear regression analysis. Each variable was first modelled separately. All variables that were significant on univariate analysis were entered simultaneously in a multivariate regression analysis model. A level of p<0.05 was considered significant. Statistical analyses were performed using SPSS software, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
PATIENT POPULATION
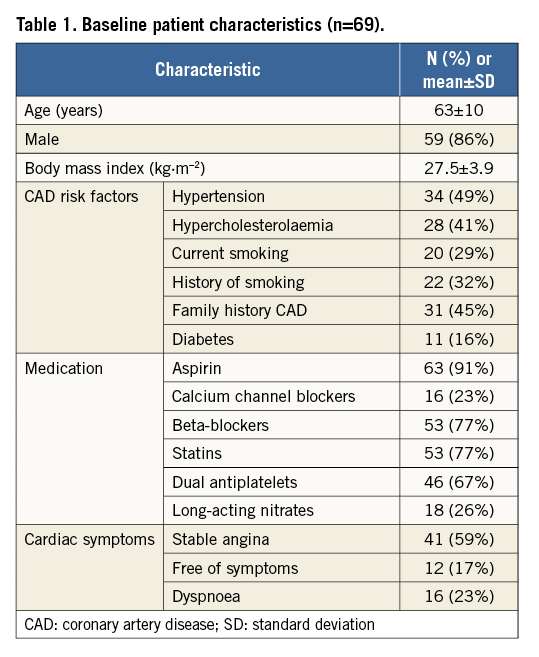
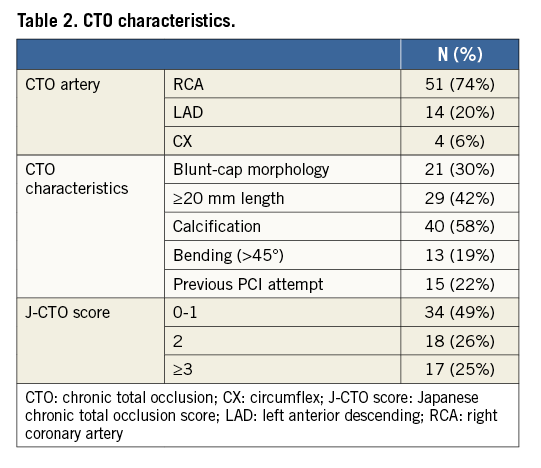
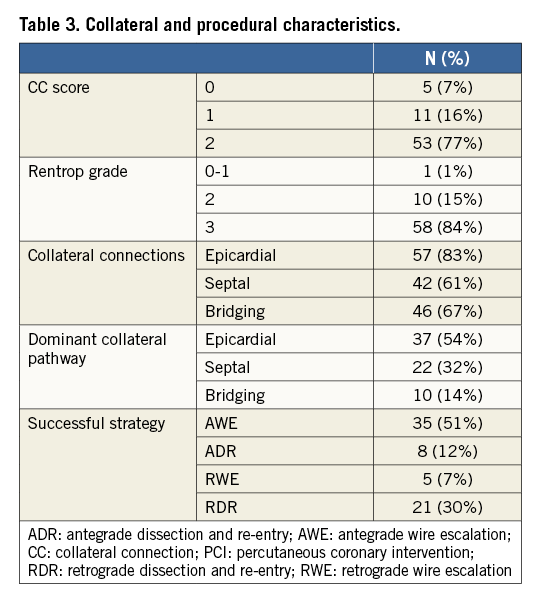
Out of 116 potentially eligible patients, sixty-nine consecutive patients (63±10 years, 86% male) analysed with cardiac PET and CMR prior to and at least 12 weeks after successful PCI CTO were included for analysis (Figure 1). Patient characteristics are shown in Table 1. The duration from PCI to follow-up imaging was 99 (96-110) days. The majority of patients were treated with PCI for the CTO vessel only (n=59, 86%), whilst ten (14%) patients were additionally treated with PCI for a non-occlusive lesion of a non-CTO coronary artery. Angiographical and procedural characteristics are listed in Table 2 and Table 3.
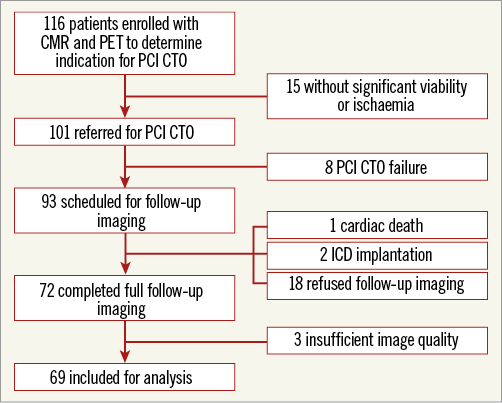
Figure 1. Patient and imaging flow chart displaying the selection procedure for follow-up imaging. CMR: cardiac magnetic resonance imaging; CTO: chronic total occlusion; ICD: implantable cardioverter defibrillator; PET: positron emission tomography; PCI: percutaneous coronary intervention
MYOCARDIAL BLOOD FLOW
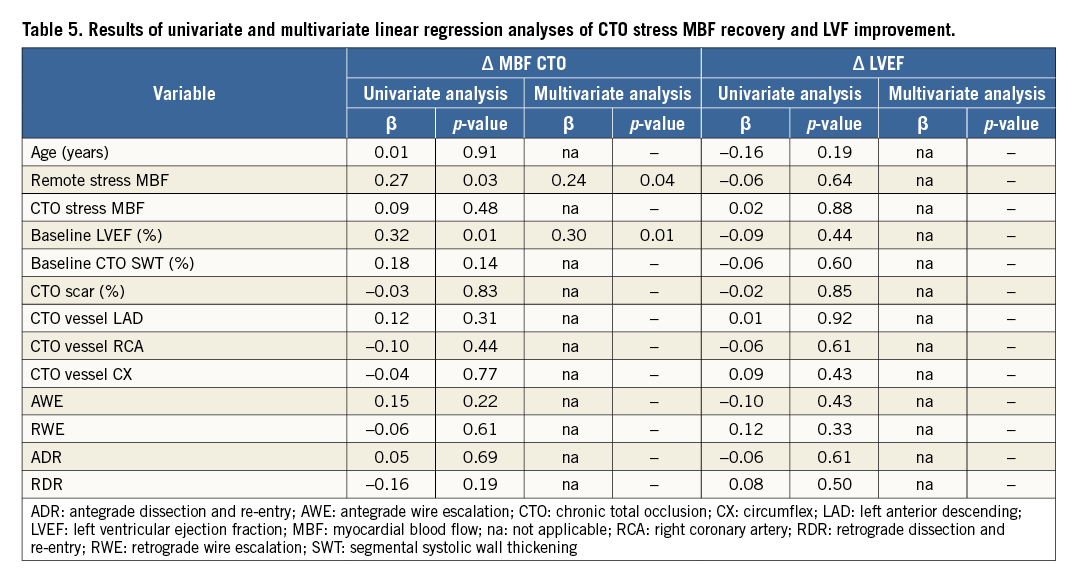
Results of PET perfusion before and after PCI CTO are shown in Table 4. MBF during rest and stress at baseline were significantly lower in the CTO territory as compared with remote (both p<0.001). After PCI, MBF during stress increased by 1.18±0.79 and 0.19±0.58 mL·min–1·g–1 in CTO and remote territories, respectively (Figure 2). The increase in remote stress MBF persisted after exclusion of patients with additional non-CTO vessel PCI (n=59, 2.64±0.70 vs. 2.81±0.81 mL·min–1·g–1, p=0.04). Mean CFR of the CTO area was significantly lower compared with CFR remote at baseline (p<0.001), and resolved after PCI (p=0.62) (Table 4). Univariate and multivariate analysis showed that baseline remote stress MBF and baseline LVEF were independently related to change in stress MBF of the CTO territory (Table 5).
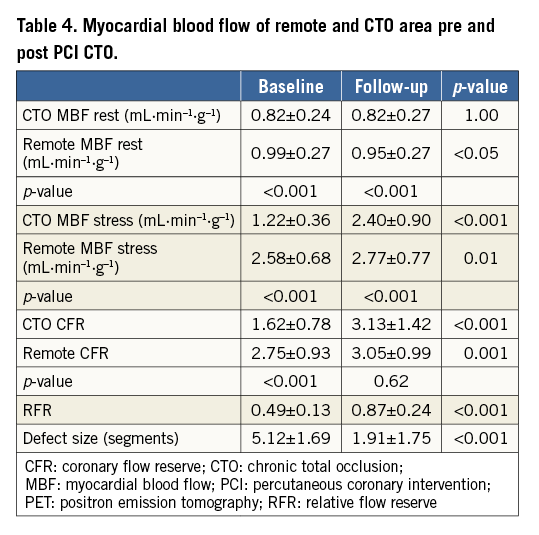
RELATIVE FLOW RESERVE AND DEFECT SIZE
RFR of the CTO territory at baseline was, on average, 0.49±0.13, and increased to 0.87±0.24 after PCI CTO (Figure 2). There was a significant reduction in defect size after PCI (5.12±1.69 vs. 1.91±1.75 myocardial segments, p<0.001) (Table 4).
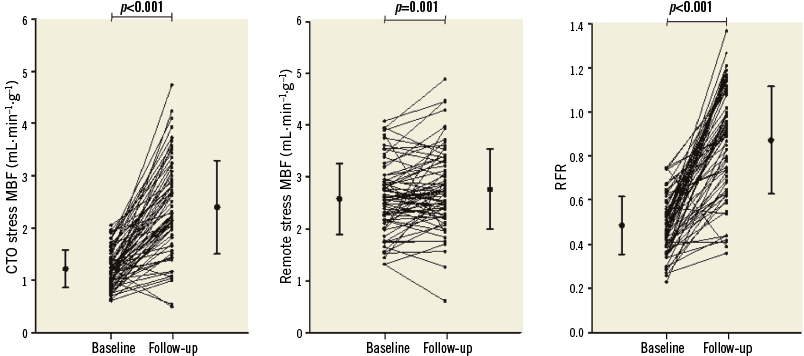
Figure 2. Stress MBF of the CTO territory, stress MBF of the remote territory, and RFR increased significantly between baseline and follow-up PET. CTO: chronic total occlusion; MBF: myocardial blood flow; RFR: relative flow reserve
LEFT VENTRICULAR FUNCTION AND VOLUME
Mean LVEDV as well as mean LVESV decreased from baseline to follow-up CMR (p=0.03 and p<0.01, respectively) (Table 6, Figure 3). LVEF increased, on average, by 1.1±4.1% after PCI CTO (p=0.01). SWT of viable segments (n=287, 26%) increased from 27.2±13.2 to 37.1±21.7% (p<0.001). Mean SWT of the CTO territory, including segments without wall motion abnormalities and segments without viability, did not improve significantly at follow-up CMR (p=0.18) (Table 6). The correlation of LGE extent with LVEF and SWT of the CTO territory is illustrated in Figure 4. Univariate analysis did not identify any predictors for change in LVEF after PCI (Table 5). Patients with pathological Q-waves in the CTO territory on baseline ECG (n=13, 19%) showed significantly more LGE in the CTO myocardial area (mean [IQR]: 13.8 [7.1;31.2] vs. 3.2 [0;10.5]%, p=0.002) and a lower baseline LVEF (37.9±9.8 vs. 48.3±10.4%, p=0.002) as compared with patients with non-Q-wave ECGs. No significant improvement in LVEF was observed among patients with pathological Q-waves in the area corresponding with the treated CTO (n=13, 37.9±9.8 vs. 38.2±11.8%, p=0.83).
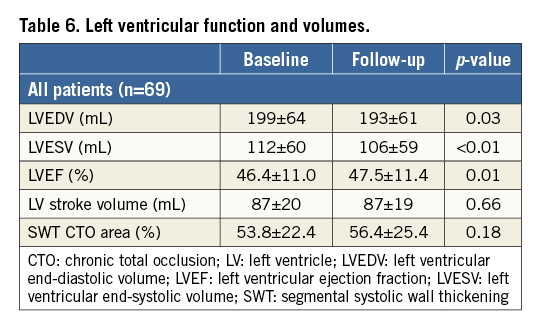
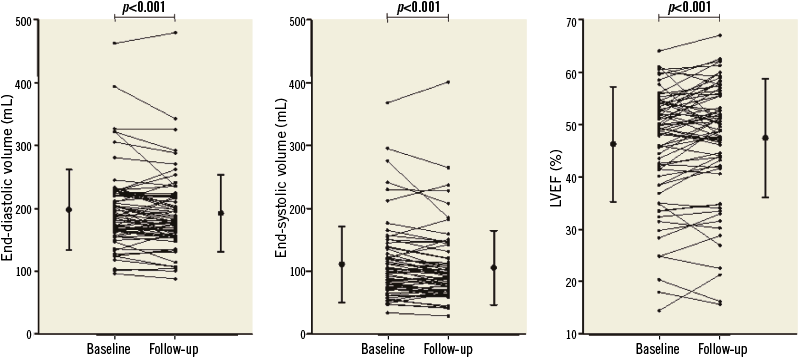
Figure 3. Left ventricular volumes and function between baseline and follow-up CMR imaging.
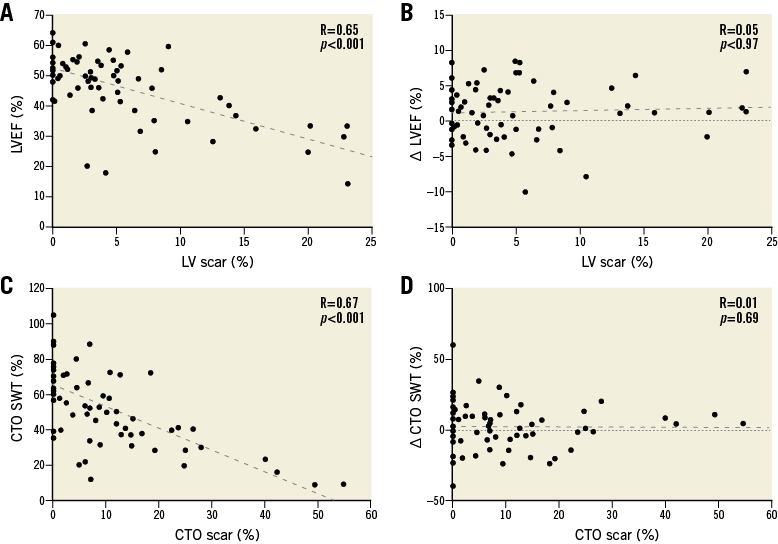
Figure 4. Scatter and Bland-Altman plots illustrating the relation of left ventricular function with myocardial scar. Relationship of LVEF (A) and change in LVEF with LV scar (B). Panel C shows CTO SWT in relation to scar in the CTO area, whilst panel D illustrates that there is no correlation between change of segmental systolic wall thickening and scar in the CTO area at follow-up. CTO: chronic total occlusion; LV: left ventricle; LVEF: left ventricular ejection fraction; SWT: segmental systolic wall thickening
RELATION BETWEEN MYOCARDIAL PERFUSION AND FUNCTION
Figure 5 displays the moderate relationship of baseline stress MBF with LGE extent and regional wall thickening as well as the absent relationship of stress MBF change with LGE extent and change of regional wall thickening in the CTO territory at follow-up. An example of a patient with normalisation of stress MBF and a significant improvement in LVEF due to PCI CTO is illustrated in Figure 6A and Figure 6B; the majority of patients did not show a significant increase of LVEF, although stress MBF normalised (Figure 6C, Figure 6D).
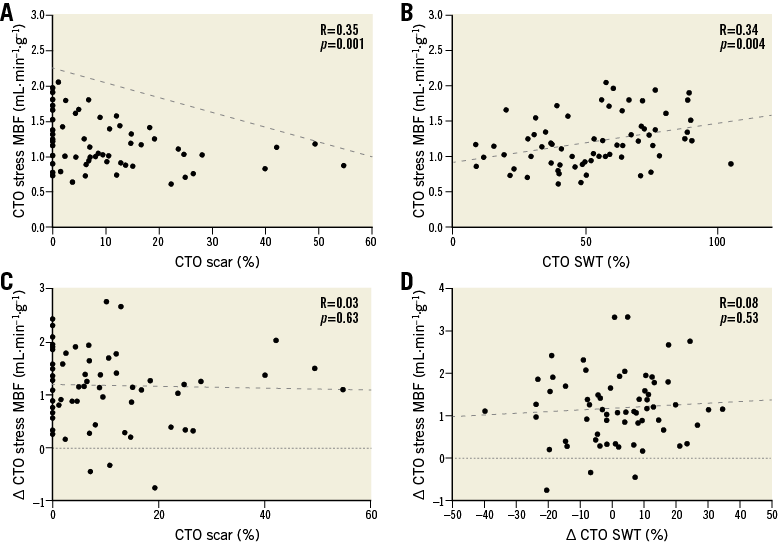
Figure 5. Scatter and Bland-Altman plots illustrating the relation of CTO stress myocardial blood flow with regional left ventricular function and scar. Relationship of CTO stress MBF with CTO scar (A) and segmental systolic wall thickening of the CTO territory (B). Panel C illustrates that there is no correlation between change in CTO stress MBF and scar of the CTO territory, whilst panel D shows the lack of a relationship between change in CTO stress MBF and change in segmental systolic wall thickening of the CTO territory at follow-up. CTO: chronic total occlusion; MBF: myocardial blood flow; SWT: segmental systolic wall thickening
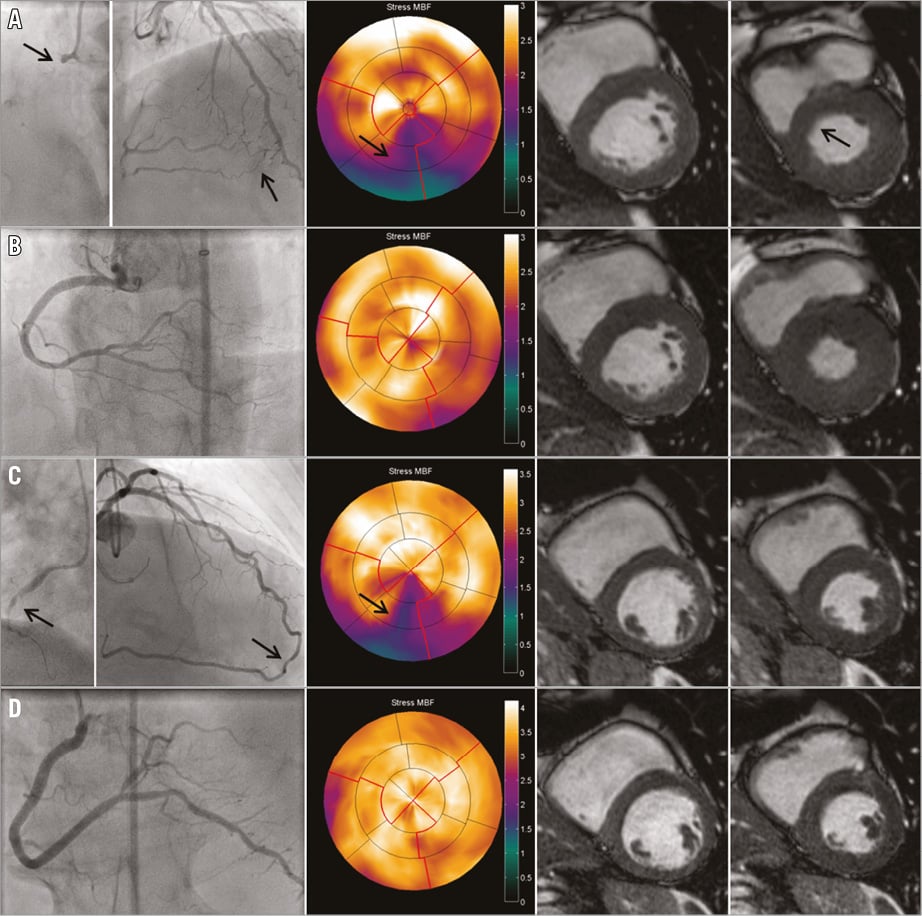
Figure 6. Two examples of patients illustrating the potential effect of successful percutaneous revascularisation on left ventricular perfusion and function. Panel A shows a patient with a proximal CTO RCA (first arrow) and septal collateral filling from the LAD to distal RCA (second arrow). On PET perfusion there is a clear perfusion defect of the inferior and inferoseptal wall (third arrow). CMR end-diastolic and end-systolic cine images revealed hypokinesia of the inferoseptal wall (fourth arrow). After successful percutaneous revascularisation (B), stress MBF on PET normalised and the LVEF increased due to improved inferoseptal wall motion. Panel C illustrates a patient with a CTO RCA (first arrow: proximal cap) and clear perfusion defect on stress PET (third arrow) in the presence of very well-developed collaterals (second arrow). End-diastolic and end-systolic cine CMR showed a moderately reduced LVEF (right images). This patient was successfully treated with PCI with a good angiographic result (D). Stress MBF normalised, but LVEF did not improve three months after PCI CTO. CMR: cardiac magnetic resonance imaging; CTO: chronic total occlusion; LAD: left anterior descending artery; LVEF: left ventricular ejection fraction; MBF: myocardial blood flow; PCI: percutaneous coronary intervention; PET: positron emission tomography; RCA: right coronary artery
Discussion
The present prospectively conducted study evaluated the recovery of MBF and LVF after successful percutaneous revascularisation of a CTO in a patient population with documented ischaemia and viability. The main results demonstrated that stress MBF increased substantially, resulting in a decrease in the preprocedural perfusion defect size of more than three myocardial segments in the vast majority of cases. In contrast, there was only a minor improvement in LVEF of 1.1%, which was not related to restoration of stress MBF.
PATIENT SELECTION
The present study population consisted of patients with stable angina, dyspnoea, as well as asymptomatic patients. All included patients were evaluated using LGE-CMR and perfusion PET to assure the presence of viable myocardium and ischaemia before referral for PCI CTO. This strategy has previously been suggested to select patients eligible for revascularisation legitimately3,6. The present results highlight that such a diagnostic workup provides a patient selection where a significant reduction in ischaemic burden can be anticipated after PCI, without a profound impact on LV function.
MYOCARDIAL BLOOD FLOW
Stress MBF in the myocardial CTO area prior to PCI was severely impaired, which was illustrated by a significantly compromised CFR and an absolute stress MBF reduction of about 50%, as compared with normally perfused myocardium within the same patient (remote). This is in line with previous studies, where a high prevalence of inducible perfusion deficits was observed in patients with a CTO2,5. The presence of a CTO together with a perfusion deficit is a significant independent predictor for major adverse cardiac events (MACE)12,13. On top of a marked increase in absolute myocardial perfusion, a profound reduction in defect size was observed after PCI in the present study. According to the standardised 17-segment model of the American Heart Association, three segments equals 17.5% of the myocardium, and should be classified as prognostically relevant12,14. Multivariate analysis identified that preserved remote vasodilator response as well as preservation of LVEF acted as independent predictors for recovery of stress MBF after PCI.
Interestingly, remote stress MBF also increased significantly. Collateralisation to the distal CTO vessel keeps the myocardium viable, but extracts flow from the donor myocardium itself. Upon restoration of antegrade flow to the previous CTO territory, the collateral flow regresses virtually instantaneously, thereby augmenting a hyperaemic response to the donor artery. This phenomenon has previously been confirmed by an increase in fractional flow reserve of the donor vessel after successful PCI CTO4,15.
LEFT VENTRICULAR FUNCTION
Previous studies using left ventricular angiography have shown mainly favourable results regarding LVF after revascularisation of high-grade stenosis as well as CTOs in the circumstance of persisting patent vessels16-18. On the other hand, more recent studies investigating the recovery of LVEF after PCI CTO using CMR imaging have demonstrated conflicting results6,19,20. Discrepancies between these studies are probably related to the method of LVF assessment, patient population characteristics, a predominantly retrospective study design, and relatively small sample sizes. The present study was conducted prospectively, selected patients on the presence of viable myocardium over the full range of baseline LVF, and is the largest in this subject group. There was no significant improvement of SWT in the CTO territory after successful PCI CTO, but a significant slight increase in LVEF combined with some extent of reverse remodelling was observed at three-month follow-up. Pujadas et al observed that EDV increased six months after a failed procedure, suggesting negative LV remodelling over time in the presence of a persisting CTO19. Therefore, it could be hypothesised that the process of negative LV remodelling would progress if a CTO were to be left untouched.
As expected, baseline LVEF was significantly related to the extent of LV scar, yet functional recovery (LVEF and SWT) was not. By selecting only viable but dysfunctional myocardial segments, however, a significant improvement in SWT was observed. These findings are in line with previous results exploring the relationship between viability and functional recovery after revascularisation21. Myocardial dysfunction is a prerequisite for the ability to show functional recovery due to reperfusion. Functional recovery is, therefore, an unsuitable surrogate for revascularisation benefit when baseline LVEF is preserved, and the effect of revascularisation in the present study may be underestimated. On the other hand, no significant improvement in LVEF was observed in the few patients (n=13) with previous Q-wave myocardial infarction in the CTO territory, suggesting that pathological Q-waves may be a robust marker for the absence of viability and thus limited functional benefit from revascularisation in CTO patients, although the sample size of the present study may be insufficient to draw definite conclusions on these findings. Interestingly, univariate analysis did not identify any predictors of LVEF recovery. This is in contrast to the results of a study by Werner et al and the Total Occlusion Study of Canada (TOSCA) which revealed that baseline LV dysfunction was an independent predictor for improvement in LVEF after revascularisation of CTO22,23, although the absolute increase in LVEF in the TOSCA study was comparable with the results of the present study. Moreover, there was no correlation between improvement of stress MBF and functional recovery. This suggests that, in the presence of mainly viable tissue, reduced myocardial function is predominantly provoked by myocardial damage (scar) rather than repetitive ischaemia.
Limitations
Imaging at follow-up was not performed in patients after a failed procedure. Therefore, it remains unclear what the effect of a failed procedure would have been on MBF and LVEF. The risks of a CTO procedure should be included in the decision-making process for PCI CTO. In addition, the present study did not incorporate assessment of potential recurrent luminal narrowing or occlusion at the time of follow-up imaging. This may have resulted in an underestimation of LVF improvement since continued vessel patency is a major determinant for functional recovery. Moreover, CMR imaging was performed approximately three months after revascularisation; however, an ongoing improvement in LVF can be observed up to three years after revascularisation of CTO24. The short follow-up period could have hampered the magnitude of regional and global recovery of LVF after PCI CTO. Furthermore, the proportion of LAD CTO lesions in the present study was relatively low as compared with previous studies6,20,23. The LAD normally comprises a large amount of the LV myocardial mass and a more profound impact on LVEF may have been observed in case of even distribution of CTO among the three major coronary arteries.
Conclusions
The vast majority of CTO patients selected based on the presence of ischaemia and viable myocardium showed a significant improvement in stress MBF and a reduction in ischaemic zone of >3 myocardial segments after successful percutaneous revascularisation. Re-opening a CTO improved LVEF only slightly but could potentially protect from negative LV remodelling. These results, combined with recently reported high success rates for PCI CTO, suggest that patients should be selected based on the presence of viable myocardium and ischaemia rather than on anatomical CTO characteristics.
| Impact on daily practice Evidence regarding patient benefit of PCI CTO is lacking. The present study showed that there is a considerable decrease of ischaemic burden and a slight significant increase in LVEF after successful PCI CTO. These results suggest that revascularisation should be considered in patients with a documented CTO. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

