
Abstract
Aims: The aim of this study was to explore the relationships between ischaemic burden and changes in absolute myocardial perfusion following chronic coronary total occlusion (CTO) percutaneous coronary intervention (PCI).
Methods and results: A total of 193 consecutive patients underwent [15O]H2O positron emission tomography prior to and three months after successful CTO PCI. Change in perfusion defect size, quantitative hyperaemic myocardial blood flow (MBF) and coronary flow reserve (CFR) within the CTO area were compared among patients with limited (0-1 segment, N=15), moderate (2-3 segments, N=61) and large (≥4 segments, N=117) perfusion defects. Median reductions in defect size were 1 [0-1], 2 [1-3], and 4 [2-5] segments in patients with a limited, moderate and large defect (all comparisons p<0.01). Hyperaemic MBF and CFR improved significantly regardless of baseline defect size (overall between groups p=0.45 and p=0.55). After stratification of patients to a low, intermediate or high tertile according to baseline hyperaemic MBF or CFR levels, changes in hyperaemic MBF and CFR after CTO PCI were comparable between tertiles (overall p=0.75 and p=0.79).
Conclusions: Major reductions in ischaemic burden can be achieved following CTO PCI, with more defect size reduction in patients with a larger perfusion defect, whereas hyperaemic MBF and CFR improve significantly irrespective of their baseline values or perfusion defect size.
Visual summary. Major reductions in ischaemic burden can be achieved following CTO PCI. More defect size reduction in patients with a larger baseline perfusion defect. Significant hyperaemic MBF improvement irrespective of its baseline values.
Introduction
Chronic coronary total occlusions (CTO) signify a detrimental impact on long-term prognosis1,2. This negative effect has been related to the extent of perfusion defect size associated with the CTO lesion evaluated with myocardial perfusion imaging3. A few studies have demonstrated that marked ischaemia is present in the vast majority of patients with a CTO regardless of well-developed collaterals4,5. Optimal medical therapy is the first-line treatment in patients with a CTO; however, its effect on ischaemic burden is limited and merely aimed at symptom relief6. Prior studies showed substantial improvements in myocardial perfusion after CTO percutaneous coronary intervention (PCI) similar to PCI of haemodynamically significant non-occluded lesions7. In addition, a beneficial effect of revascularisation on long-term prognosis in patients with a CTO and moderate-to-severe ischaemia was previously suggested8,9. Current international guidelines therefore recommend CTO revascularisation in patients with a marked ischaemic burden10. However, data on the effectiveness of CTO PCI on ischaemia reduction in patients with various degrees of ischaemic burden are lacking. The aim of the present study was to determine the impact of CTO PCI on the relief of different extents of ischaemia as quantified with [15O]H2O positron emission tomography (PET).
Methods
STUDY DESIGN AND PARTICIPANTS
Prospectively recruited consecutive patients presenting with a CTO in the Amsterdam UMC location Vrije Universiteit Amsterdam between 2013 and 2018 were eligible for inclusion if the presence of ischaemia was evaluated with [15O]H2O PET perfusion imaging prior to and following successful CTO PCI. Patients were rescheduled for [15O]H2O PET imaging at least three months after revascularisation. Exclusion criteria were pregnancy and contraindications for adenosine administration. A documented history of myocardial infarction (MI) was reported according to patient files or if pathological Q-waves were present on the electrocardiogram11. Left ventricular ejection fraction was assessed during clinical work-up by echocardiography or cardiac magnetic resonance imaging. The study was approved by the Amsterdam UMC location Vrije Universiteit Amsterdam Medical Ethics Review Committee, and all patients provided written informed consent.
ANGIOGRAPHIC CHARACTERISTICS
Angiographic characteristics were evaluated on a monoplane cardiovascular X-ray system (Allura Xper FD 10/10; Philips Healthcare, Best, the Netherlands). CTOs were defined as a luminal occlusion on invasive coronary angiography for an estimated time of ≥3 months with Thrombolysis In Myocardial Infarction (TIMI) flow grade 0-1. Collaterals supplying the vascular territory of the CTO were graded according to the collateral connection (CC) score12. CTO PCI was performed according to the hybrid approach and successful revascularisation was defined as TIMI flow grade 3 and <30% diameter stenosis7. Side branch loss (≥2 mm) was scored and cardiac biomarkers were obtained if periprocedural MI was suspected, which was defined according to the Fourth Universal Definition of Myocardial Infarction11.
POSITRON EMISSION TOMOGRAPHY
Patients underwent a dynamic emission scan at rest and during hyperaemia by administration of intravenous adenosine (140 μ·kg−1·min−1)4. Rest and hyperaemic myocardial blood flow (MBF, in mL·min−1·g-1) and coronary flow reserve (CFR) as the ratio of hyperaemic to rest MBF were measured in the CTO myocardial area. The standardised 17-segment model of the American Heart Association was used for left ventricular segmentation. The perfusion defect size associated with the CTO was defined by the number of myocardial segments in which hyperaemic MBF was below 2.3 mL·min−1·g−1 and <75% compared to hyperaemic MBF in a normal reference vascular territory7,13. The perfusion defect size at baseline was classified per patient as limited (0-1 segments), moderate (2-3 segments) or large (≥4 segments). Furthermore, all patients were stratified in tertiles according to hyperaemic MBF and CFR levels at baseline.
STATISTICAL ANALYSIS
Normally distributed data are presented as mean±standard deviation and analysed with a paired samples t-test, independent samples t-test or one-way ANOVA test. Non-normally continuous data are presented as median (interquartile range) and analysed with a Wilcoxon signed-rank test, Mann-Whitney U test, or Kruskal-Wallis test. Categorical variables are presented as numbers and percentages and analysed with the Fisher’s exact test. In case of >2 groups and the overall p-value being p<0.05, pairwise comparisons were made between groups using a Bonferroni correction to correct for multiple testing. Changes in perfusion indices within the CTO area after PCI were compared between patients with limited, moderate and large perfusion defects at baseline. In addition, change in hyperaemic MBF was compared between patients classified in the lowest (≤1.00 mL·min−1·g−1), intermediate (1.01-1.39 mL·min−1·g−1) and highest (≥1.40 mL·min−1·g−1) tertiles of hyperaemic MBF at baseline. Accordingly, change in CFR was compared between patients in the lowest (≤1.30), intermediate (1.31-1.77) and highest (≥1.78) tertiles of CFR at baseline. A univariate generalised linear model was used for linear regression analyses to find predictors for change in perfusion defect size after CTO PCI. Variables were entered into the multivariable analysis if the p-value was ≤0.10 in the univariable analysis. Receiver operating curve analyses were used to identify the optimal baseline perfusion defect size threshold for subsequent ≥1 and ≥2 segment defect size reduction after CTO PCI. A level of p<0.05 was considered significant. Statistical analyses were performed using SPSS software, Version 22.0 (IBM Corp., Armonk, NY, USA) and MedCalc software 11.6.0.0 (MedCalc, Mariakerke, Belgium).
Results
PATIENT POPULATION
Between 2013 and 2018, 193 patients (84% male, mean age 63±11 years) were successfully treated with CTO PCI and were rescheduled for follow-up PET imaging, whereas 10 patients (out of 203, 5%) could not be successfully treated. Patients with failed CTO PCI did not undergo follow-up PET imaging and were excluded from analysis. Clinical and angiographic characteristics of the successfully treated patients are shown in Table 1 and Supplementary Table 1. A CTO lesion with TIMI flow 0 or 1 was observed in 153 (79%) and 40 (21%) patients, respectively. In patients with a large perfusion defect, the CTO was located more often in the left anterior descending artery (LAD) compared to patients with a moderate perfusion defect (p=0.02). Collaterals had a CC score of 2 in 134 (69%) patients, with no differences between groups (overall p=0.59). In 13 (7%) patients, a second procedure was needed to revascularise the CTO successfully. Spontaneous MI or unplanned myocardial revascularisation did not occur in any patient between serial PET imaging. After follow-up PET imaging and clinical evaluation, three patients were additionally treated with PCI of the CTO artery. In two of these three patients, residual ischaemia in the CTO territory was caused by a significant stenosis distal of the former CTO lesion, and in one patient residual ischaemia was present due to a compromised side branch at the site of the revascularised CTO.
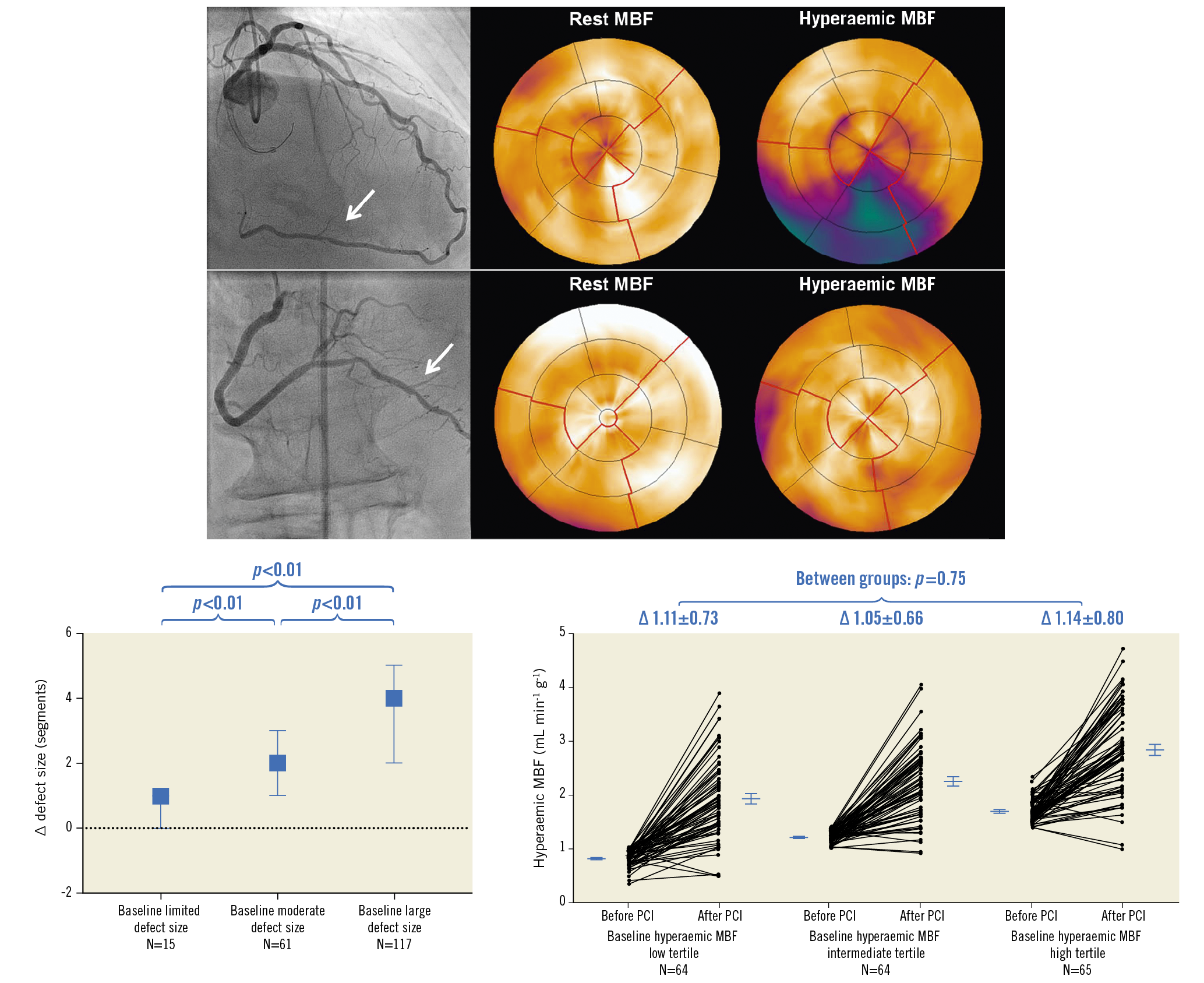
CHANGE IN MYOCARDIAL PERFUSION AFTER CTO PCI ACCORDING TO BASELINE DEFECT SIZE
The median number of days between baseline PET and CTO PCI, and between CTO PCI and follow-up PET, was 37 (22-59) and 103 (94-122), respectively. Change of rest MBF and CFR could not be analysed in three patients due to one failed rest scan at baseline and two failed rest scans at follow-up. Myocardial perfusion findings at baseline and follow-up are shown in Table 2. A total of 178 out of 193 patients (92%) had a perfusion defect size of ≥2 segments. Hyperaemic MBF and CFR at baseline were significantly lower in a large perfusion defect compared with a limited (both p<0.01) or moderate perfusion defect (both p<0.01). In patients with failed CTO PCI, the perfusion defect was larger and both hyperaemic MBF and CFR were lower in comparison with the successfully treated population (Supplementary Table 2). In the successfully treated population, myocardium subtended by CTO lesions with TIMI flow grade 0 had lower hyperaemic MBF and CFR values compared to CTO lesions with TIMI flow grade 1 (Supplementary Table 3). Overall changes in rest MBF, hyperaemic MBF and CFR after PCI were 0.03±0.22 mL·min−1·g−1, 1.10±0.73 mL·min−1·g−1 and 1.29±1.03, respectively. These changes were comparable between patients with various defect sizes (Figure 1) and after revascularisation of a CTO lesion with TIMI flow grade 0 or 1, respectively. An overall median residual perfusion defect of 1 [0-2] segment was found after CTO PCI. The median decrease in defect size after PCI was 3 [1.5-4.5], and was significantly different among patients with a limited, moderate or large perfusion defect at baseline (1 [0-1], 2 [1-3], 4 [2-5], respectively, all comparisons p<0.01) (Figure 2). Changes of all perfusion indices after CTO PCI were non-significantly different between patients with CC score 2 collaterals and patients with CC 0-1 collaterals supplying the myocardium subtended by a CTO (Supplementary Table 4). Side branch loss and periprocedural MI did not result in hampered recovery of any perfusion outcome (all p>0.05). Case examples are displayed in Figure 3.
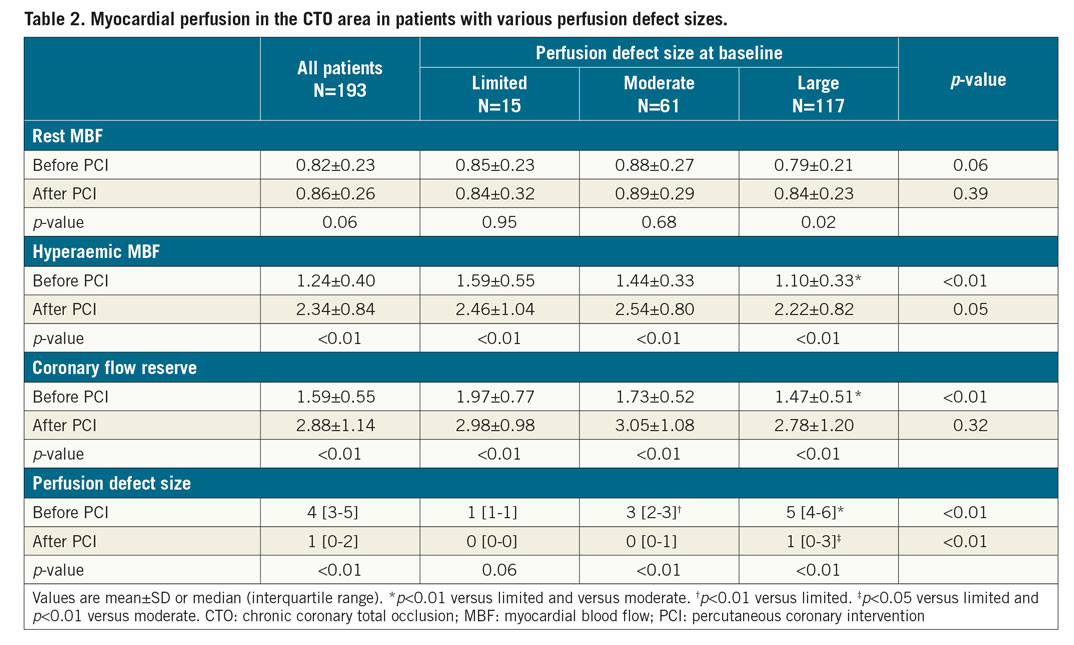
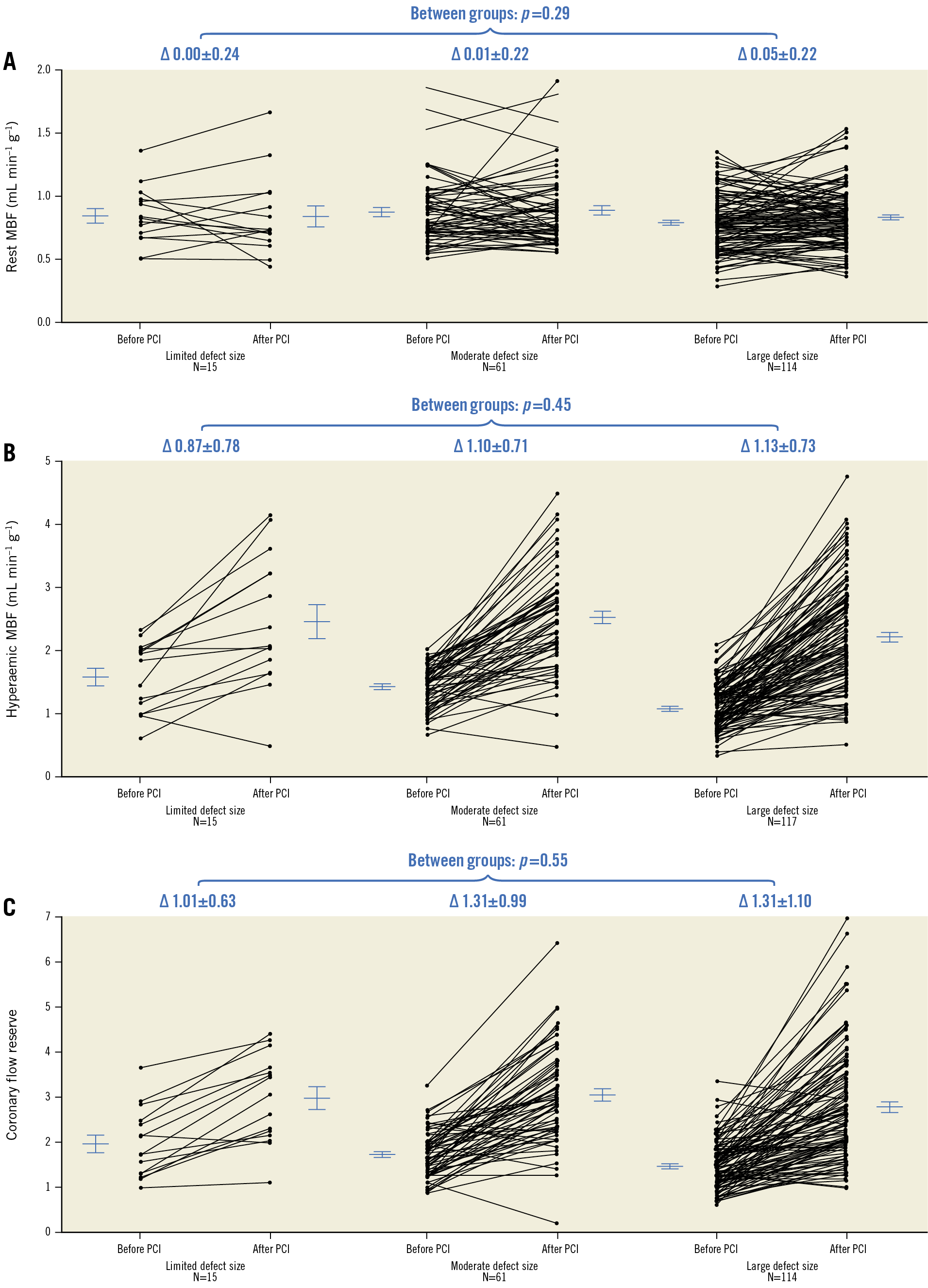
Figure 1. Per patient paired measurements. Per patient paired measurements (lines) of rest MBF (A), hyperaemic MBF (B) and CFR (C) in patients with a limited, moderate and large perfusion defect at baseline. Horizontal lines and error bars are mean and standard error of the mean. ∆: change; CFR: coronary flow reserve; MBF: myocardial blood flow; PCI: percutaneous coronary intervention
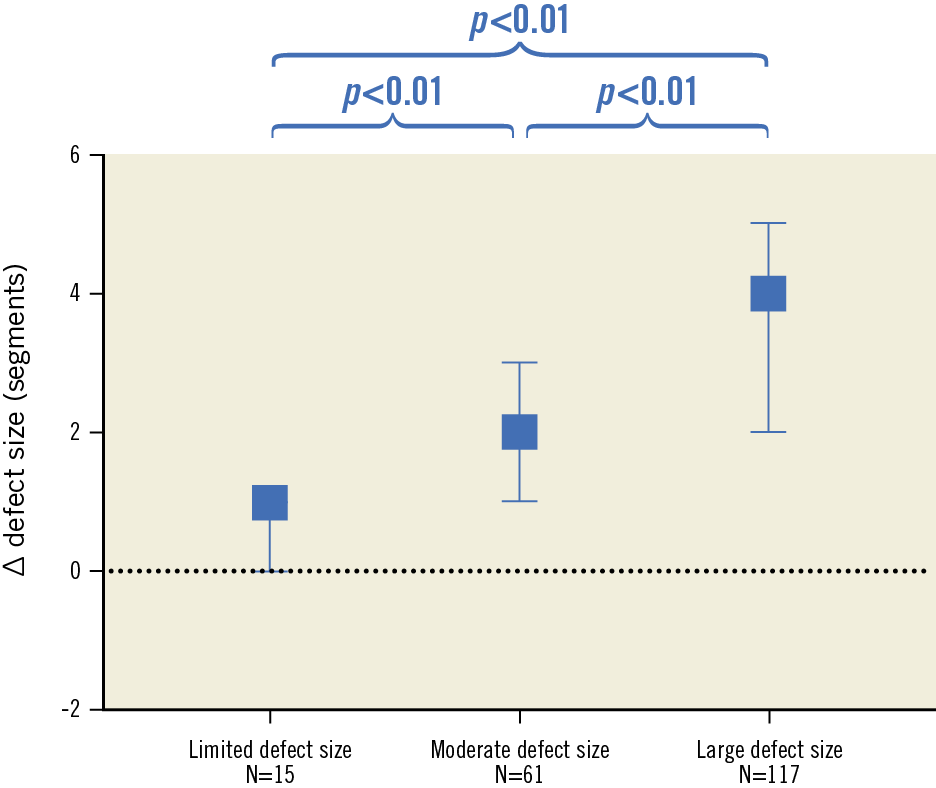
Figure 2. Change in defect size after CTO PCI in patients with a limited, moderate and large perfusion defect. Boxes and error bars are median (interquartile range). ∆: change; CTO: chronic coronary total occlusion; PCI: percutaneous coronary intervention
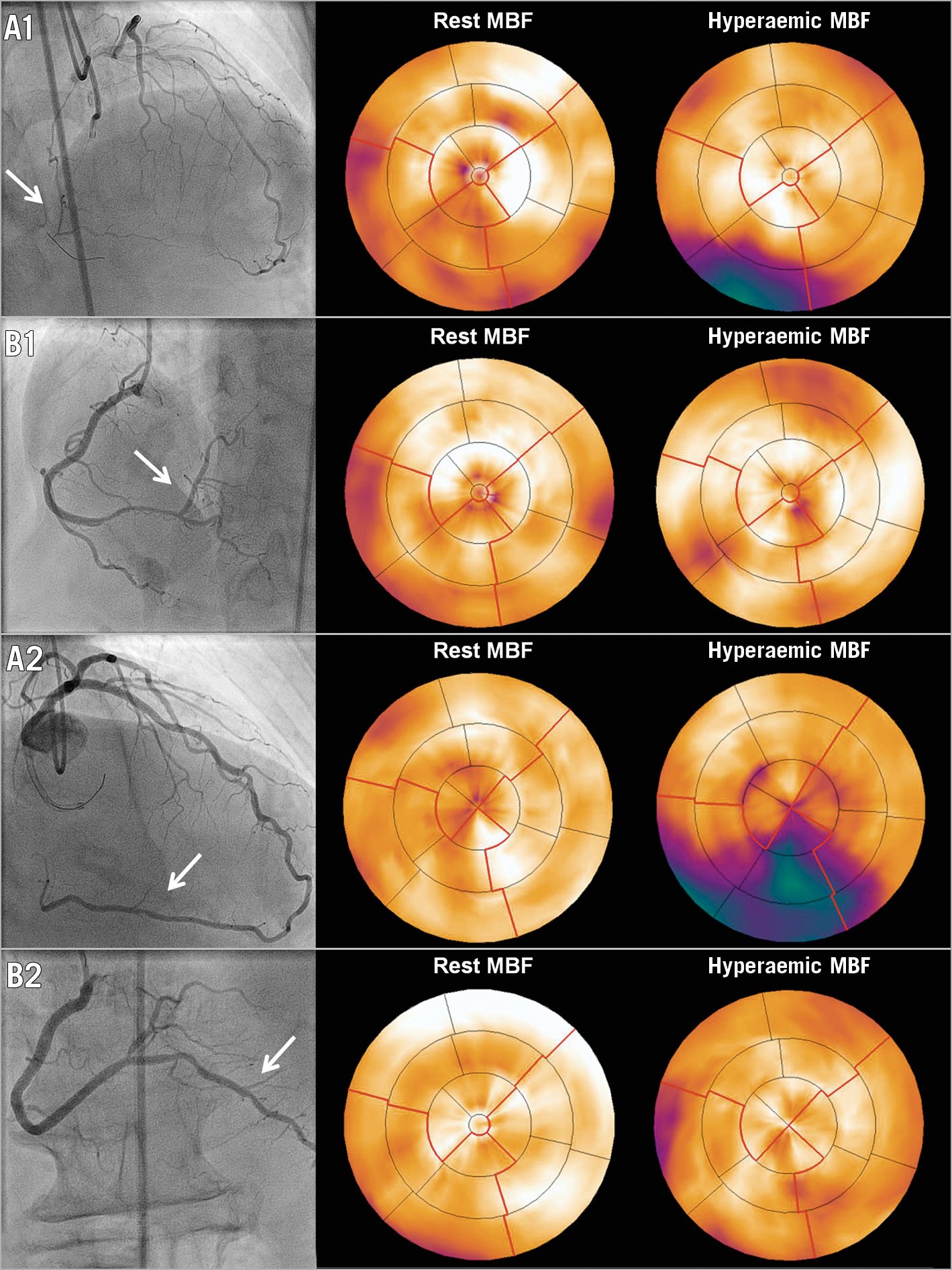
Figure 3. CTO PCI in a patient with a limited and a large perfusion defect size. A1) The vascular territory (arrow) subtended by a CTO in the right coronary artery and [15O]H2O PET perfusion showing a limited associated perfusion defect. After successful CTO PCI, antegrade blood flow was restored (arrow, B1) with restoration of PET perfusion after three months. A2) The vascular territory (arrow) distal of a CTO in the right coronary artery and [15O]H2O PET perfusion displaying a large associated perfusion defect. Successful CTO PCI (arrow, B2) led to a major reduction in defect size. CTO: chronic coronary total occlusion; MBF: myocardial blood flow; PCI: percutaneous coronary intervention; PET: positron emission tomography
PREDICTORS OF CHANGE IN PERFUSION DEFECT SIZE AFTER CTO PCI
In multivariable analysis, the CTO artery was a significant predictor of defect size reduction after CTO PCI, with relatively more reduction if the CTO was located in the LAD (Supplementary Table 5). Receiver operating curve analyses identified a perfusion defect of ≥3 segments as the optimal threshold to predict ≥1 segment (83% sensitivity and 50% specificity) and ≥2 segment (92% sensitivity and 56% specificity) defect size reduction.
CHANGE IN HYPERAEMIC MBF AFTER CTO PCI ACCORDING TO BASELINE HYPERAEMIC MBF
Baseline hyperaemic MBF in the CTO area in patients within the low, intermediate and high tertiles of hyperaemic MBF was 0.82±0.15, 1.21±0.11 and 1.69±0.22 mL·min−1·g−1, respectively. Baseline characteristics of patients within the three tertiles are shown in Supplementary Table 6. After PCI, hyperaemic MBF increased significantly (paired data within each tertile p<0.01) to 1.93±0.75, 2.25±0.69 and 2.84±0.82 mL·min−1·g−1 at follow-up (low tertile vs intermediate tertile p=0.048, low tertile vs high tertile p<0.01, intermediate tertile vs high tertile p<0.01). Change in hyperaemic MBF was comparable among the groups (overall p=0.75) (Figure 4A).
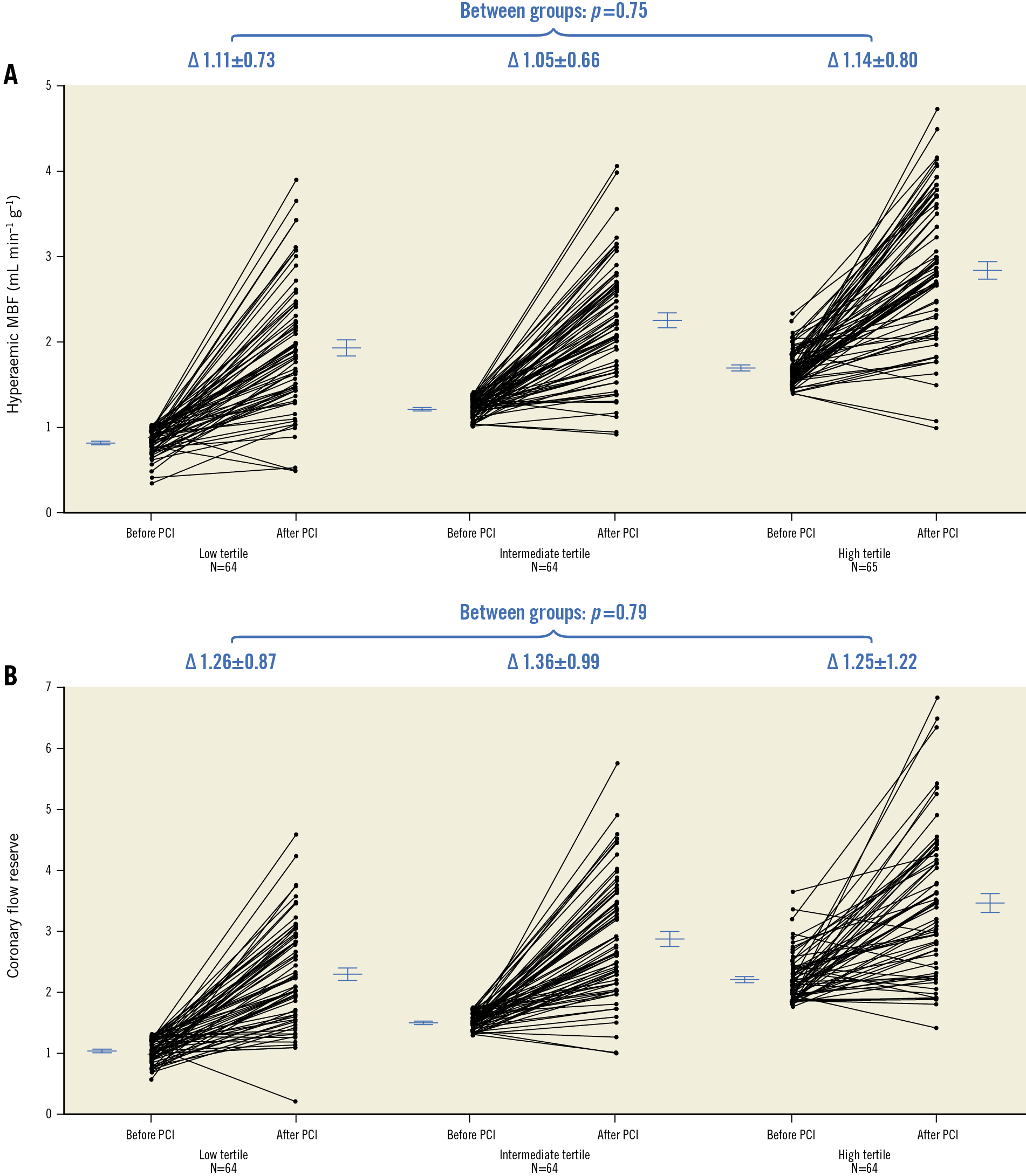
Figure 4. Per patient paired measurements. A) Per patient paired measurements (lines) of hyperaemic MBF in the CTO area in patients stratified to the low, intermediate and high tertiles according to hyperaemic MBF values at baseline. B) Per patient paired measurements (lines) of CFR in the CTO area in patients stratified to the low, intermediate and high tertiles based on CFR values at baseline. Horizontal lines and error bars are mean and standard error of the mean. ∆: change; CFR: coronary flow reserve; CTO: chronic coronary total occlusion; MBF: myocardial blood flow; PCI: percutaneous coronary intervention
CHANGE IN CFR AFTER CTO PCI ACCORDING TO BASELINE CFR
The CFR in the CTO area at baseline in patients in the low, intermediate and high CFR tertiles was 1.04±0.19, 1.52±0.14 and 2.21±0.40, respectively, and increased significantly after PCI (paired data within each tertile p<0.01) to levels of 2.29±0.86, 2.89±1.00 and 3.47±1.24, respectively, at follow-up (all comparisons between groups p<0.01). Baseline characteristics of patients within the three tertiles are shown in Supplementary Table 7. The change in CFR was comparable between the groups (overall p=0.79) (Figure 4B).
Discussion
The main findings can be summarised as follows: 1) at baseline, 92% of patients had a perfusion defect size of ≥2 segments (>10% of the left ventricle); 2) greater reduction in defect size after CTO PCI was achieved in patients with a larger perfusion defect at baseline; 3) hyperaemic MBF and CFR levels before PCI were more severely impaired in patients with a larger perfusion defect; and 4) increases in hyperaemic MBF and CFR were significant and not related to baseline values or perfusion defect size before PCI.
EXTENT OF THE PERFUSION DEFECT AND DEPTH OF QUANTITATIVE MBF IN PATIENTS WITH A CTO
Ninety-two percent of patients had a perfusion defect of ≥2 segments (>10% of the left ventricle), which is associated with a negative impact on long-term prognosis and is considered a clinical indication for CTO PCI3,10. All patients had hyperaemic MBF and CFR levels well below the cut-off values for ischaemia as defined by [15O]H2O PET (hyperaemic MBF: 2.3 mL·min–1·g–1, and CFR: 2.5)13. These levels of hyperaemic MBF and CFR were impaired irrespective of the visually assessed collateral status in patients. In addition, hyperaemic MBF and CFR levels were more severely hampered in a larger perfusion defect despite the equal distribution of well-developed collaterals across various perfusion defect sizes, questioning the causal relationship between the amount of ischaemia and collateral recruitment. However, the accuracy to quantify the collateral blood supply capacity by visual assessment is limited12. From a pathophysiological point of view, it could be assumed that collateral blood supply is less pronounced in patients with a larger perfusion defect size and lower MBF. Alternatively, one could argue that arteriogenesis of (un)recognised collaterals is by definition limited. As such, if collateral blood supply has to be distributed over a greater amount of myocardial tissue at risk subtended by a CTO, as is likely in patients with a larger perfusion defect size, this distribution may lead to lower (hyperaemic) MBF and CFR levels.
PERFUSION DEFECT SIZE REDUCTION AFTER CTO PCI
Recently, the 12-month outcomes of the randomised EuroCTO trial were revealed14. The aim of this trial was to compare the change in health status and prognostic outcomes between patients with a CTO randomised to CTO PCI and those randomised to optimal medical therapy. All non-occlusive lesions in patients with multivessel disease were treated before randomisation to establish a genuine comparison. Greater improvements in the Seattle Angina Questionnaire subscales quality of life and angina frequency were observed after CTO PCI, whereas major adverse cardiac events were comparable between the two groups. The prognostic outcomes at three-year follow-up in the EuroCTO trial are eagerly awaited. In the present study, CTO PCI resulted in a median defect size reduction of three segments which equals 17.5% of the left ventricle myocardium and may be classified as prognostically relevant according to prior literature8,9. A CTO located in the LAD was a significant predictor for greater defect size reduction. This finding is consistent with prior literature demonstrating that the LAD in general subtends a larger myocardial area than the other epicardial vessels15. In 2011, Safley et al reported that ischaemia detection could enhance the recognition of patients with a high potential for major ischaemic burden reduction after CTO PCI, which in turn might contribute to improved long-term survival8. Importantly, the additional value of the current study to the work of Safley et al is that: 1) CTO PCI was performed according to the current standards of the hybrid approach, 2) absolute myocardial perfusion was measured by [15O]H2O PET being the gold standard for non-invasive myocardial perfusion imaging, and 3) patients were prospectively approached for follow-up PET after a fixed period of time. At follow-up, a median defect size of 1 (0-2) segment was observed. Recovery of myocardial perfusion in patients with a residual perfusion defect could have been hampered due to the presence of microvascular dysfunction and lower baseline hyperaemic MBF which predispose a patient to end up with lower follow-up hyperaemic MBF levels16,17. Secondly, restenosis in the CTO artery could have stayed unrecognised in some patients due to a lack of re-invasive coronary angiography at the time of follow-up PET. It should be noted, however, that a median residual defect size of 0 to 1 segment (<10% of the left ventricle) among all patient subgroups suggests that CTO PCI generally results in no or limited residual ischaemia.
IMPROVEMENTS IN HYPERAEMIC MBF AND CFR AFTER CTO PCI
Patients with lower baseline hyperaemic MBF had suffered more frequently from prior MI in the CTO territory and less often had a preserved left ventricular ejection fraction, which are both risk factors for microvascular dysfunction16,17. However, hyperaemic MBF and CFR improved significantly after CTO PCI irrespective of their baseline values and perfusion defect size before revascularisation. Collaterals with lower CC scores supplying the CTO artery have been related to more pronounced endothelial and smooth muscle dysfunction in myocardium subtended by a CTO, and could potentially limit recovery of absolute myocardial perfusion after CTO PCI18. In the present study, however, hyperaemic MBF and CFR improved significantly regardless of visually assessed collateral status. While prior studies have suggested that perfusion defect size reduction might be prognostically relevant, the prognostic validation of change in hyperaemic MBF and CFR after revascularisation is lacking8,9. Theoretically, CTO PCI might be prognostically beneficial even in patients with a limited perfusion defect size due to the significant improvements in hyperaemic MBF and CFR19.
Limitations
Although marked ischaemia is found in the vast majority of patients with a CTO, asymptomatic patients with a limited perfusion defect have not been included in the current analysis due to the lack of a clinical indication for CTO PCI4,5. Change in myocardial perfusion in patients with failed CTO PCI could not be evaluated as follow-up PET imaging was not performed to limit radiation exposure. As cardiac biomarkers were not systematically obtained before and after CTO PCI, the incidence of periprocedural myocardial injury might have been underestimated. In addition, the potential relationship between change in myocardial perfusion and change in cardiac enzymes after CTO PCI could not be adequately explored, and the influence of periprocedural MI on recovery of myocardial perfusion remains unclear. A gradual increase of myocardial perfusion up to three months after coronary revascularisation has been reported previously20. In the present study, myocardial perfusion findings after CTO PCI (e.g., residual defect sizes) might have improved further if the follow-up duration of three months had been prolonged.
Conclusions
Significant reduction in ischaemia can be achieved with CTO PCI across all levels of ischaemic burden as expressed by perfusion defect size, with greater defect size reduction in patients with a larger perfusion defect at baseline. Patients with a CTO and a larger perfusion defect have more severely impaired hyperaemic MBF and CFR levels. Notwithstanding, hyperaemic MBF and CFR improve significantly after recanalisation of CTO irrespective of their baseline values or perfusion defect size before PCI. Ischaemia detection by quantitative PET could be used as an effective tool to recognise patients with a high potential for marked ischaemia reduction after CTO PCI.
|
Impact on daily practice There is a lack of evidence regarding the effects of CTO PCI on relief of different levels of ischaemic burden. The present study showed that significant reduction in ischaemia can be achieved with CTO PCI across all levels of ischaemic burden. Greater defect size reduction was observed in patients with a larger perfusion defect at baseline, whereas increases in hyperaemic MBF and CFR were significant but independent of their baseline values or perfusion defect size. These results demonstrate that quantitative PET can be an effective tool to select patients with high potential for marked ischaemia reduction. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

