Abstract
Aims: Albumin is a marker of frailty. Scarce data are available on correlations between frailty-related parameters and outcomes in patients undergoing TAVI. This study sought to evaluate the relation between albumin and mortality in TAVI candidates.
Methods and results: A total of 150 patients (mean age 81±6 years) undergoing TAVI were included in the study. Patients with pre-procedural albumin >4 g/dl (>40 g/L) (n=71) were compared to those ≤4 g/dl (≤40 g/L) (n=79). The cut-off value of 4 g/dl (40 g/L) was based on the mean value of albumin in the patients included in the study. During a mean follow-up of 2.1 years the survival rate was 72%. Patients in both groups had similar baseline characteristics. The 2.1-year mortality was higher in the low albumin group compared with the normal albumin group (35% vs. 19%, p=0.01). Multivariate analysis indicated that low pre-procedural albumin was independently associated with a more than twofold increase in 2.1-year all-cause mortality (p=0.01, HR=2.28; 95% CI: 1.17-4.44). Low post-procedural serum albumin remained a strong parameter correlated with all-cause mortality (HR=2.47; 95% CI: 1.28-4.78; p<0.01).
Conclusions: Baseline albumin can be used as a simple tool that correlates with survival after TAVI. Low albumin is an important parameter associated with all-cause mortality after the procedure.
Abbreviations
AKI: acute kidney injury
AVA: aortic valve area
BMI: body mass index
CABG: coronary artery bypass graft
CAD: coronary artery disease
COPD: chronic obstructive pulmonary disease
CVA: cerebrovascular accident
eGFR: estimated glomerular filtration rate
LVEF: left ventricular ejection fraction
PVD: peripheral vascular disease
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
TIA: transient ischaemic attack
VARC2: Valve Academic Research Consortium 2
Introduction
Transcatheter aortic valve implantation (TAVI) is an effective therapy for severe aortic stenosis in elderly patients with high surgical risk1,2. Due to ageing of the population, the number of potential TAVI candidates has significantly increased3 over the last two decades. However, outcomes of patients undergoing TAVI are variable, and predicting which patients are most likely to benefit from this procedure is a major challenge for the Heart Team.
The concept of frailty has been recognised as an important factor in the risk stratification of patients undergoing invasive procedures4, especially in elderly patients with cardiovascular disease5-7. Frail patients have been shown to have worse outcome after such interventions; however, there is no current consensus regarding the definition of frailty. Risk assessment of patients with aortic stenosis is complex: several tools are available to the Heart Team for pre-procedural evaluation of TAVI candidates, including bedside clinical evaluation8, the logistic EuroSCORE and EuroSCORE II9-11, the Society of Thoracic Surgeons (STS) score12, the Ambler and age, creatinine, ejection fraction (ACEF) scores and various biological markers. These risk stratification tools evaluate predominantly the patients’ comorbidities and to a lesser extent the degree of vulnerability and disability which characterise the frail elderly patient. For this purpose, several frailty scores were tested, such as the ISAR score and SHERPA score13. All these scores may help, predominantly in pre-procedural risk stratification of each patient considered for TAVI, but correlation with outcomes, especially long-term mortality after TAVI, is poor14. Additional tools for simple pre-procedural assessment of TAVI candidates are needed15-17. Serum albumin was proposed by VARC2 in their most recent consensus document to assess frailty. However, there are scarce data available regarding the specific correlation between albumin, as a marker of frailty, and outcomes in patients undergoing TAVI.
We undertook the present study to evaluate the relationship between periprocedural levels of serum albumin and mortality in patients undergoing TAVI.
Methods
PATIENT POPULATION
All patients with severe symptomatic aortic stenosis and high or prohibitive operative risk who underwent successful TAVI by femoral approach in our institution from October 2009 to August 2012 were included in this retrospective study. Transcatheter aortic valve implantation was performed using a CoreValve prosthesis (Medtronic, Minneapolis, MN, USA) or an Edwards SAPIEN XT valve (Edwards Lifesciences, Irvine, CA, USA). All patients underwent pre-procedural assessment in order to detect significant coronary artery disease, and revascularisation was performed if deemed necessary. None of the patients had simultaneous PCI and TAVI intervention. All patients underwent transthoracic echocardiography for the assessment of prosthetic valve function following valve implantation.
DATA COLLECTION
All data were retrospectively collected from electronic patient records and included medical history, clinical status, echocardiographic measurements, biological data, procedural information and clinical status at 30 days after TAVI. The clinical and procedural outcomes were adjudicated according to VARC2 definitions (Appendix). Survival data were obtained up to 1st October 2013 from the National Statistics Department and represent all-cause mortality in the study population, reported after 30 days during the follow-up. The study was approved by the local institutional ethics committee.
ALBUMIN-BASED ANALYSIS
Serum albumin was chosen for assessment as a marker of frailty, according to VARC2 frailty criteria. “Pre-procedural albumin” represents the value of serum albumin assessed within the 24 hours prior to the intervention. In this setting, baseline albumin was analysed both as a continuous variable and using a cut-off value of 4 g/dl (40 g/L), representing the mean value in the population and dichotomising patients into low and normal baseline albumin groups.
After the procedure, albumin levels were assessed daily during the in-hospital period. The lowest value of serum albumin during the first seven days after the procedure was considered as the “post-procedural albumin”. Post-procedural albumin was analysed using a cut-off value of 3.25 g/dl (32.5 g/L), representing the mean value in the population, and dichotomising patients into low and high post-procedural albumin groups.
Periprocedural parameters are the expression of TAVI-related variations and express the difference between the pre-procedural value and post-procedural value – presented as the “delta” value.
Baseline renal function was assessed using creatinine to estimate GFR by the MDRD formula18, and we considered a value below 30 mL/min/1.73 m2 as severely reduced renal function.
The residual SYNTAX score was assessed for all patients with significant CAD and revascularisation prior to the TAVI procedure, using Version 2.11 of the SYNTAX score calculator (http://www.syntaxscore.com/) to quantify the residual CAD19. SYNTAX score was calculated by an experienced interventional cardiologist blinded to the patients’ clinical data.
STUDY ENDPOINTS
The primary endpoint of the study was to evaluate the correlation between baseline serum albumin and all-cause mortality in TAVI patients. As a secondary endpoint we evaluated the significance of low post-procedural albumin following TAVI.
STATISTICAL ANALYSIS
Data are presented as mean±standard deviation if normally distributed or as median if not normally distributed. Continuous variables were tested with the Kolmogorov-Smirnov test for normal distribution. Categorical variables are given as frequencies and percentages. For continuous variables, a Student’s t-test was performed for comparison between two groups. A chi-square test was used for analysis of categorical variables. We performed univariate Cox proportional hazard model analysis to examine the association of albumin and other parameters with cumulative one-year and 2.1-year mortality and to evaluate the impact on the clinical outcome. In the multivariate Cox regression model, we have entered those parameters with a p-value below 0.05 in univariate analysis. The proportionality of hazards assumption was checked by the log-log plot method and we confirmed that the proportionality assumption for a covariate was satisfied when curves did not intersect. Survival according to the level of baseline albumin and post-procedural albumin was determined using the Kaplan-Meier method. The log-rank test was used to determine statistical differences in terms of survival. Statistical significance was assumed when the null hypothesis could be rejected at p<0.05. All p-values are results of two-sided tests. Statistical analyses were conducted using IBM SPSS Statistics software, Version 20 (IBM Corp., Armonk, NY, USA). The investigators initiated the study, had full access to data and analysed the data, and wrote the manuscript. All authors vouch for the data and analysis.
Results
The study included 150 consecutive TAVI patients with an average age of 81±6 years, the majority of whom were women (60%). The mean left ventricular (LV) ejection fraction in the population was 54±12%, and the baseline peak and mean aortic valve gradients were 74±25 mmHg and 45±15 mmHg, respectively. The study population demonstrated a mean baseline albumin level of 4±0.4 g/dl (40±4 g/L). The distribution of baseline serum albumin among study patients is shown in Figure 1. Of the study cohort, 79 patients (53%) had a baseline serum albumin level ≤4 g/dl (≤40 g/L) (low baseline albumin group). The remaining 71 patients (47%) served as the comparison group of patients with normal baseline serum albumin (normal albumin group). The baseline characteristics of the two groups are shown in Table 1. Notably, patients with low baseline albumin had significantly less dyslipidaemia, but no significant differences were observed regarding other baseline characteristics.
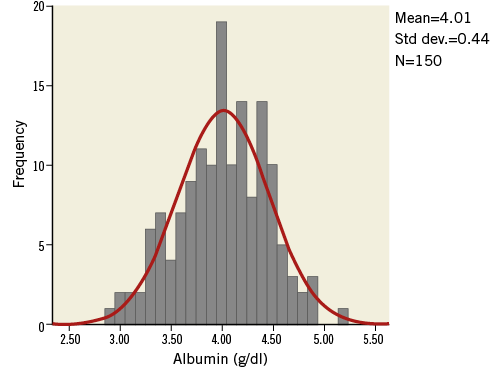
Figure 1. Distribution of baseline serum albumin. Normal distribution of baseline albumin in the study population (Kolmogorov-Smirnov Z=1.01; p-value=0.25).
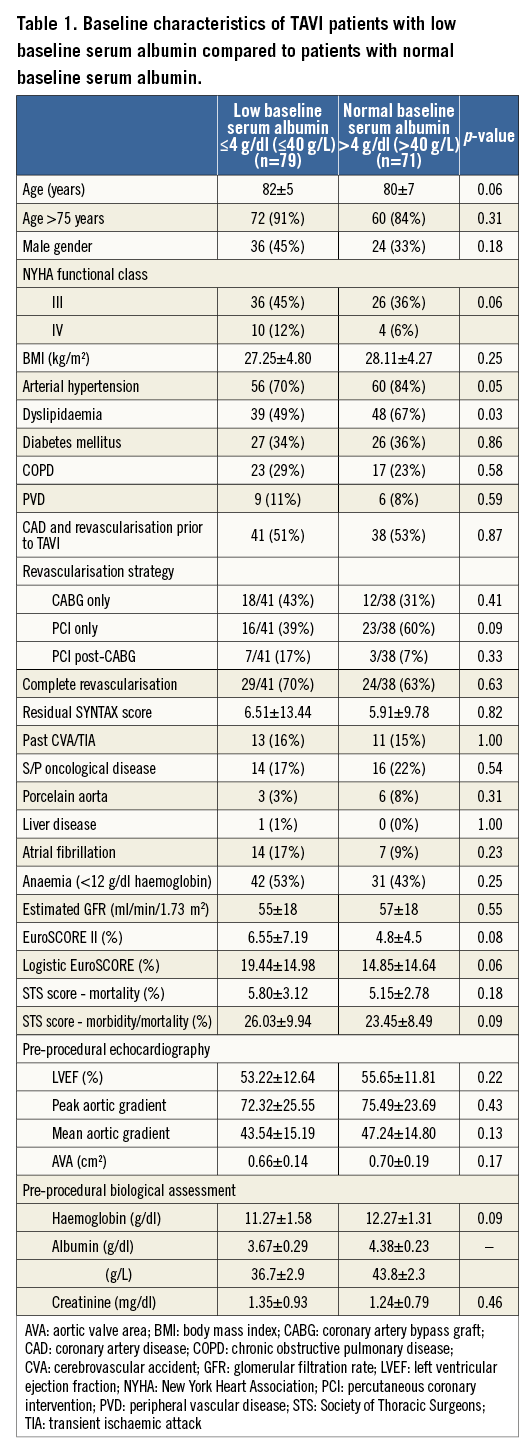
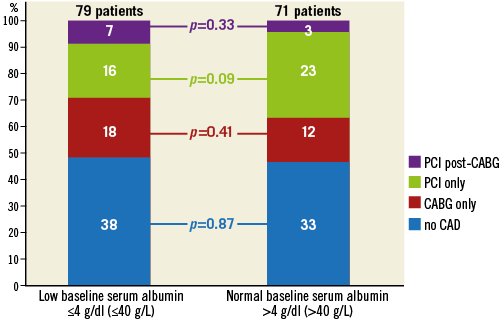
No significant differences in procedural and post-procedural characteristics were observed between the two groups (Table 2). All patients with significant coronary artery disease (CAD) had revascularisation prior to TAVI. The rate of complete revascularisation and the revascularisation strategy (PCI only, CABG only and PCI post-CABG) were similar between groups (Table 1). In patients with CAD and incomplete revascularisation (due to unfavourable coronary anatomy or absence of proven significant ischaemia) the residual SYNTAX score was similar between groups (p=0.82) (Table 1, Appendix Figure 1).
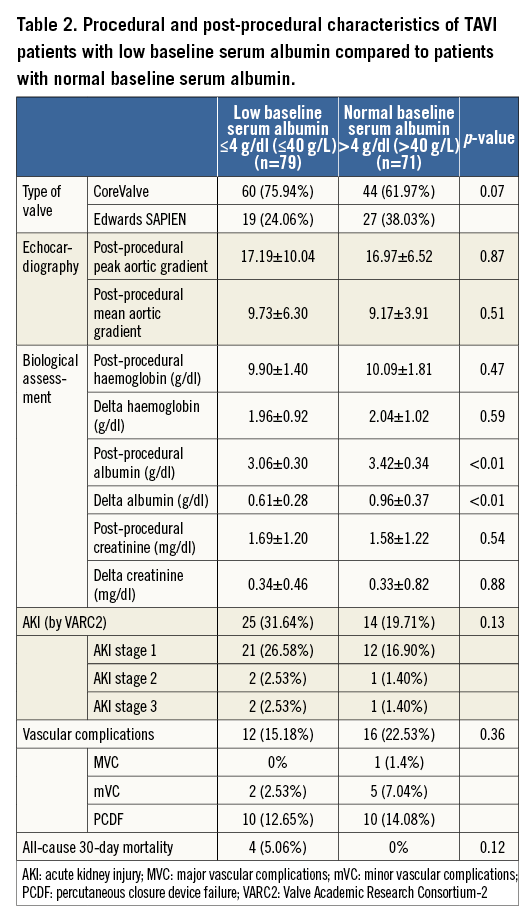
Among the entire study population, four deaths occurred during the first month after TAVI (three cardiac and one non-cardiac). Periprocedural vascular event rates did not differ between the two groups (p=0.36). Acute kidney injury (AKI) occurred overall in 26% of the patients. The majority developed the mild form (AKI stage 1: 22%; AKI stage 2: 2%; AKI stage 3: 2%), and no significant differences were noted between patients with low compared to normal baseline albumin (p=0.13). Except for three patients who were already on chronic renal dialysis prior to the TAVI procedure, no other patients required dialysis during hospitalisation. Both groups demonstrated a post-procedural drop of serum albumin (Table 2).
Baseline albumin and all-cause mortality
ONE-YEAR FOLLOW-UP
At one year, 22 out of 150 patients had died (14.66%). Four patients (5.63%) died in the normal baseline albumin group compared with 18 patients (22.78%) in the low baseline albumin group (p<0.01). In the univariate analysis, a low baseline albumin was associated with a more than fourfold increase in all-cause mortality (HR=4.56; 95% CI: 1.54-13.48, p<0.01). Additional factors shown to be associated with mortality in the univariate Cox analysis were: presence of atrial fibrillation, ejection fraction, mean aortic valve gradient, and baseline renal function. A strong correlation was noted between low baseline albumin and one-year all-cause mortality (HR=4.46; 95% CI: 1.51-13.21, p<0.01) even after adjustment for renal function.
TWO-YEAR FOLLOW-UP
During a mean follow-up of 2.1 years, a total of 42 patients died (28%), 14 (19.71%) in the normal baseline albumin group and 28 (35.44%) in the low baseline albumin group. Kaplan-Meier survival analysis of the whole population showed that patients with low baseline albumin experienced significantly lower survival rates at 2.1 years of follow-up as compared with those with normal baseline albumin (Figure 2). Thus, at 2.1 years of follow-up, the survival rates were 65% among patients with low baseline albumin as compared with 80% among patients with normal albumin (log-rank p-value=0.01).
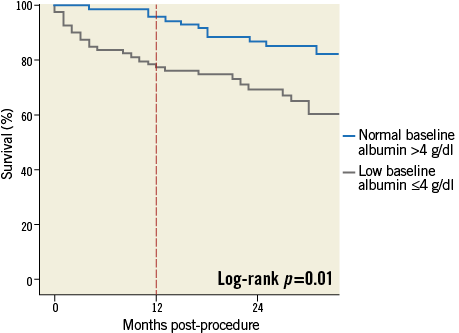
Figure 2. Kaplan-Meier long-term survival probability in patients with low baseline albumin (≤4 g/dl) /(≤40 g/L) versus patients with normal baseline albumin (>4 g/dl)/ (>40 g/L) (65% vs. 80%; log-rank p=0.01). However, the main difference in mortality between low and normal baseline albumin groups occurs at one year (23% vs. 6%; log-rank p<0.01).
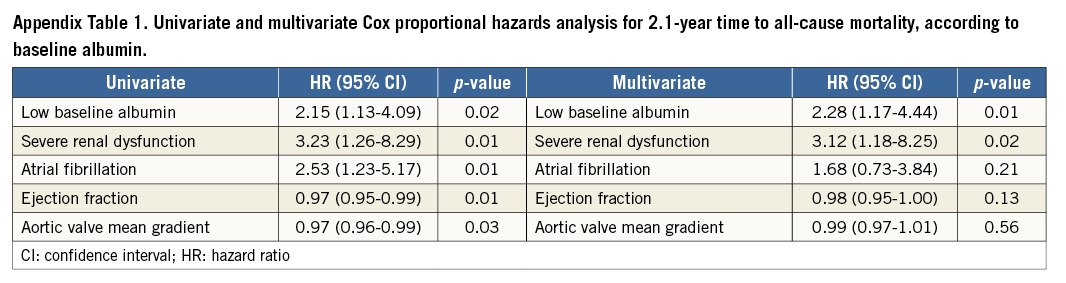
In the multivariate analysis, patients with low baseline albumin experienced a more than twofold increase in 2.1-year mortality risk (Figure 3). Furthermore, multivariate analysis showed that each 1 g/dl (10 g/L) increment in baseline albumin was independently associated with a significant 56% reduction in the risk of 2.1-year mortality (p=0.02, HR=0.44; 95% CI: 0.21-0.89). In the multivariate Cox proportional hazards analysis only low baseline albumin and baseline severe renal dysfunction were independently associated with all-cause mortality in the population (Figure 3, Appendix Table 1).
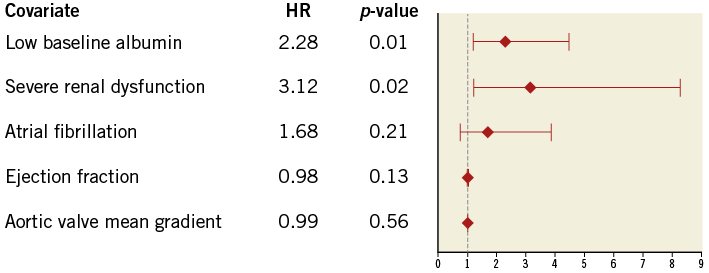
Figure 3. Multivariate Cox proportional hazards analysis for time to all-cause mortality. In the multivariate analysis, patients with low baseline albumin (≤4 g/dl) experienced a more than twofold increase in 2.1-year mortality risk (HR=2.28; 95% CI: 1.17-4.44, p=0.01); the presence of severe renal dysfunction was also independently associated with long-term mortality (HR=3.12; 95% CI: 1.18-8.25, p=0.02).
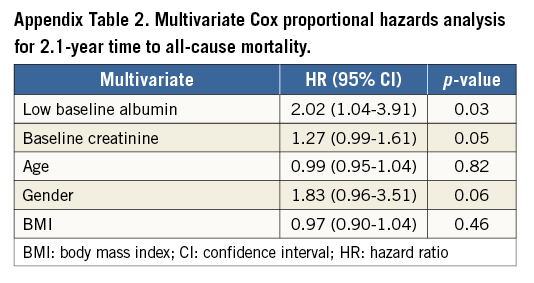
In a separate multivariate analysis, low baseline albumin remained independently associated with all-cause mortality (HR=2.02; 95% CI: 1.04-3.91, p=0.03), patients with low albumin proving to have a twofold increase of risk, after adjusting for creatinine, age, gender and body mass index (BMI) (Appendix Table 2).
A separate analysis of post-TAVI survivors at one year showed no significant difference in all-cause mortality between those with low baseline albumin compared to normal baseline albumin (83.8% vs. 83.6%; log-rank p=0.93) (Figure 2).
PERIPROCEDURAL ALBUMIN VARIATION
A post-procedural serum albumin decrease was noted throughout the entire study population. Both groups experienced a drop in serum albumin (Table 2). The variation, calculated as an absolute value (the “delta albumin”), was significantly different between groups (p<0.01), and depended upon the baseline albumin in the linear regression analysis (p<0.01). The low baseline albumin group had a drop of 0.6 g/dl albumin (6 g/L) compared with 0.9 g/dl (9 g/L) in the normal baseline albumin group.
In the multivariate Cox proportional hazards analysis no significant correlation was found between the variation of albumin and long-term mortality (p=0.69).
POST-PROCEDURAL ALBUMIN AND ALL-CAUSE MORTALITY
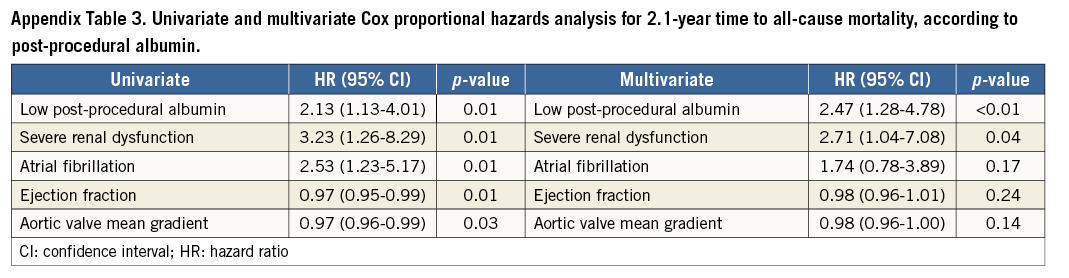
Patients with a low post-procedural albumin had significantly lower survival rates during the 2.1-year follow-up as compared with those with high post-procedural albumin. Thus, at 2.1-year follow-up the survival rate was 65% among patients with low post-procedural albumin as compared with 80% among patients with high post-procedural albumin (log-rank p-value=0.01). The multivariate analysis at 2.1 years showed that each 1 g/dl (10 g/L) decrease in post-procedural albumin was independently associated with a significant 62% increase in the risk of all-cause mortality (HR=0.38; 95% CI: 0.16-0.86, p=0.02).
A post-procedural serum albumin below 3.25 g/dl (32.5 g/L) was associated with a more than twofold increase in all-cause mortality (HR=2.47; 95% CI: 1.28-4.78, p<0.01). In the multivariate Cox proportional hazards analysis the presence of pre-procedural severe renal dysfunction remained independently associated with mortality (HR=2.71; 95% CI: 1.04-7.08, p=0.04) (Table 3, Appendix Table 3).
Discussion
The study demonstrates that low baseline albumin correlates with all-cause mortality in elderly patients undergoing TAVI, and that low post-procedural serum albumin remains an important biological parameter correlated with all-cause mortality in this population.
Frailty is an expression of vulnerability or susceptibility to disability20 and refers to a globally limited reserve to withstand stressors. It is based on the fundamental notion that homeostasis decreases with age, in parallel with a decline of physiologic reserves. Comprehensive geriatric assessment has become a well-established method to evaluate the elderly and represents the gold standard for detecting and assessing frailty. However, its use is limited because of time cost and the need for multidisciplinary expertise21.
A simplified method to detect frailty in an old patient addressed for TAVI is needed and should integrate the comorbidity, disability and vulnerability of the elderly. While the VARC2 statement mentions frailty as one of the most important patient characteristics not evaluated by classic risk scores22, and recommends serum albumin as a marker of biological reserve, limited data exist in the literature concerning the prognostic value of albumin in TAVI patients.
Albumin is quantitatively the most important plasma protein, and the synthesis and serum concentrations are regulated by a variety of factors. The decrease of albumin levels reflects a variety of conditions including malnutrition, systemic inflammation, heart failure, hepatic and renal pathologies, and it is known that among patients with chronic diseases, including heart disease, lower serum albumin levels correlate with poor outcome23,24.
Green et al25 evaluated frailty in elderly TAVI patients by a complex score composed of gait speed, grip strength, serum albumin, and activities of daily living status. This composite frailty score was shown to correlate with one-year mortality, but the authors did not report the direct correlation between albumin and mortality. Moreover, the composite frailty score evaluated in this study is complex and difficult to perform in daily practice. Moreno et al reported that mortality after TAVI has various causes26. Albumin may be a biological parameter capable of expressing all these different aetiologies. We hypothesise that albumin alone, as a marker of biological reserves, is correlated with comorbidity, disability and vulnerability – the three components recognised to be part of frailty. Based on this concept, we analysed albumin as a direct independent marker of outcome after TAVI.
We have found that a cut-off value of 4 g/dl (40 g/L) serum albumin is a very simple and useful biological parameter associated with all-cause mortality following TAVI. In patients undergoing bypass in cardiac surgery, serum albumin was shown to be a better predictor of post-procedural outcome than low BMI27. As opposed to albumin, in our study low BMI, as a marker of frailty, did not correlate with all-cause mortality.
In the present study the only significant difference in baseline characteristics between the two groups was the presence of a higher number of patients with known dyslipidaemia in the normal baseline albumin group. This may suggest that albumin, in this particular highly selected category of old patients, may be a marker of nutritional status and biological reserve, a marker for general wellbeing and not a direct marker for cardiovascular comorbidities per se.
The study offers the Heart Team a simple biological marker of frailty assessment in patients referred for TAVI, and suggests that serum albumin level might be used in addition to the commonly used scores, such as the STS and EuroSCORE II, to complete the risk profile of patients referred for TAVI.
Since low baseline albumin ≤4 g/dl (≤40 g/L) correlated with all-cause mortality, we suggest that baseline serum albumin assessment, added to other risk stratification tools, be used to help predict which patients are at increased risk of mortality after TAVI and aid in determining which patients with severe aortic stenosis are likely to have a sustained benefit following the procedure.
The drop in serum albumin after TAVI is probably a marker of periprocedural stress, and may allow quantification of the amount of TAVI-induced stress in the individual patient. Further studies are needed to elucidate this issue.
Limitations
The main limitation of the study arises from its retrospective design in a relatively small population of TAVI patients representing a single-centre experience. Due to a limited number of patients we cannot exclude the possibility of unmeasured confounders having influenced the data. The results need to be confirmed in larger, prospective, multicentre studies. Also, due to the retrospective characteristics, additional information regarding frailty was not available, so that a comparative analysis of albumin with other recognised frailty parameters was not possible.
Conclusion
The current study offers a simple objective tool to assess frailty as a part of risk stratification, helping to identify patients more likely to have sustained mortality benefit after TAVI. A low baseline albumin ≤4 g/dl (≤40 g/L) in elderly patients was associated with higher all-cause mortality. We suggest that a cut-off value of 4 g/dl (≤40 g/L) albumin may be considered in the pre-procedural assessment of elderly patients being addressed for TAVI.
| Impact on daily practice Although an important part of pre-TAVI patient evaluation, frailty assessment is currently underused in daily practice, due to the complexity of the proposed algorithms and scores. Albumin is an easily measurable biological marker that correlates with outcome in specific conditions, including heart failure and cardiac surgery. In an elderly population of severe aortic stenosis patients, baseline albumin level below 4 g/dl was independently correlated with significantly worse survival after TAVI. Thus, baseline serum albumin measurement may simplify the daily practice assessment of frailty in TAVI patients by offering the clinician a simple, objective and rapid tool for predicting the survival benefit after the procedure. |
Conflict of interest statement
A. Segev is a proctor for Medtronic Inc. and Edwards Lifesciences. V. Guetta is a proctor for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
SUPPLEMENTARY DATA
Appendix. Clinical and procedural outcomes used according to VARC2 definitions
PROCEDURAL MORTALITY
All-cause mortality within 30 days (since the index procedure hospitalisation was less than 30 for all patients included).
ALL-CAUSE MORTALITY
CARDIOVASCULAR MORTALITY
Any of the following criteria:
Death due to proximate cardiac cause (e.g., myocardial infarction, cardiac tamponade, worsening heart failure).
Death caused by non-coronary vascular conditions such as neurological events, pulmonary embolism, ruptured aortic aneurysm, dissecting aneurysm, or other vascular disease.
All procedure-related deaths, including those related to a complication of the procedure or treatment for a complication of the procedure.
All valve-related deaths including structural or non-structural valve dysfunction or other valve-related adverse events.
Sudden or unwitnessed death.
Death of unknown cause.
NON-CARDIOVASCULAR MORTALITY
Any death in which the primary cause of death is clearly related to another condition (e.g., trauma, cancer, suicide).
ACUTE KIDNEY INJURY
Analysed in the first seven days after the procedure.
Stage 1
Increase in serum creatinine to 150-199% (1.5-1.99×increase compared with baseline.
OR
Increase of >0.3 mg/dL(>26.5 mmol/L).
OR
Urine output <0.5 ml/kg/hr for >6 but <12 hrs.
Stage 2
Increase in serum creatinine to 200-299% (2.0-2.99×increase compared with baseline).
OR
Urine output <0.5ml/kg/hr for >12 but <24 hrs.
Stage 3
Increase in serum creatinine to ≥300% (>3×increase compared with baseline).
OR
Serum creatinine of ≥4.0 mg/dL (≥354 mmol/L)
with an acute increase of at least 0.5 mg/dL (44 mmol/L).
OR
Urine output <0.3 ml/kg/hr for >24 hrs.
OR
Anuria for >12 hrs.
VASCULAR ACCESS-SITE AND ACCESS-RELATED COMPLICATIONS
MAJOR VASCULAR COMPLICATIONS
Any aortic dissection, aortic rupture, annulus rupture, left ventricle perforation, or new apical aneurysm/pseudoaneurysm.
OR
Access or access-related vascular injury (dissection, stenosis, perforation, rupture, arteriovenous fistula, pseudoaneurysm, haematoma, irreversible nerve injury, compartment syndrome, percutaneous closure device failure) leading to death, life-threatening or major bleeding, visceral ischaemia, or neurological impairment.
OR
Distal embolisation (non-cerebral) from a vascular source requiring surgery or resulting in amputation or irreversible end-organ damage.
OR
The use of unplanned endovascular or surgical intervention associated with death, major bleeding, visceral ischaemia or neurological impairment.
OR.
Any new ipsilateral lower extremity ischaemia documented by patient symptoms, physical exam, and/or decreased or absent blood flow on lower extremity angiogram.
OR
Surgery for access site-related nerve injury.
OR
Permanent access site-related nerve injury.
MINOR VASCULAR COMPLICATIONS
Access-site or access-related vascular injury (dissection, stenosis, perforation, rupture, arteriovenous fistula, pseudoaneurysms, haematomas, percutaneous closure device failure) not leading to death, life-threatening or major bleeding, visceral ischaemia, or neurological impairment.
OR
Distal embolisation treated with embolectomy and/or thrombectomy and not resulting in amputation or irreversible end-organ damage.
OR
Any unplanned endovascular stenting or unplanned surgical intervention not meeting the criteria for a major vascular complication.
OR
Vascular repair or the need for vascular repair (via surgery, ultrasound-guided compression, transcatheter embolisation, or stent graft).
PERCUTANEOUS CLOSURE DEVICE FAILURE
Failure of a closure device to achieve haemostasis at the arteriotomy site leading to alternative treatment (other than manual compression or adjunctive endovascular ballooning).
Appendix Figure 1. Coronary artery disease in the low baseline albumin group compared with the normal baseline albumin group. No significant differences were noted regarding the presence of significant CAD and the strategy of revascularisation.