
Abstract
Aims: Sarcopaenia is a prevalent disease of ageing, associated with adverse clinical outcomes. We aimed to compare in-hospital adverse outcomes and overall mortality in sarcopaenic and non-sarcopaenic patients undergoing transcatheter aortic valve replacement (TAVR).
Methods and results: This was a retrospective cohort study including 602 patients who underwent TAVR. Sarcopaenia was defined as skeletal muscle mass index <55.4 cm2/m2 in males and <38.9 cm2/m2 in females obtained through pre-TAVR CT scan. Mortality, length of hospital stay, ICU admission, and Valve Academic Research Consortium (VARC)-2-defined post-TAVR complications were defined as outcomes. Study participants (mean age 80.9±8.9 years and 56.8% male) were followed for a median of 1.5 years. Two thirds of the TAVR population was sarcopaenic. In-hospital outcomes were similar in both groups; however, overall survival was worse in sarcopaenic patients (HR for mortality=1.46 [1.06-2.14], p=0.02). In a multivariable model, sarcopaenia, porcelain aorta, pre-TAVR atrial fibrillation/flutter, severe chronic kidney disease, chronic pulmonary disease, VARC-2 bleeding, acute renal failure following TAVR, and post-TAVR cardiac arrest were predictors of mortality.
Conclusions: Sarcopaenic patients had similar in-hospital clinical outcomes to non-sarcopaenic patients following TAVR which reveals TAVR safety in sarcopaenic patients. However, sarcopaenia was an independent risk factor for midterm mortality indicating its potential value in systematic evaluation of this highly comorbid population in order to decide the best treatment approaches.
Introduction
Aortic valve stenosis (AS) is the most common type of valvular heart disease in developed countries with an increasing prevalence worldwide due to the ageing population. Surgical and transcatheter valve interventions impart significant improvement in the clinical course and survival of symptomatic severe AS patients1. Transcatheter aortic valve replacement (TAVR) is currently approved for severe AS patients with intermediate, high, or prohibitive surgical risk1. Co-existent preoperative atrial fibrillation2, pulmonary disease, pulmonary hypertension, and right-sided heart failure3 are predictors of poor survival post TAVR. In particular, frailty is associated with a twofold to threefold decrease in one-year survival4.
Sarcopaenia is a newly described and prevalent geriatric syndrome that is linked to frailty5. Although the exact definition of sarcopaenia is still evolving, it is characterised by muscle wasting and decreased muscle strength and function. Sarcopaenia is an indicator of biological ageing and systemic conditions such as cachexia, chronic malnutrition, and inflammatory states and accordingly is associated with poor clinical outcomes5. Radiologically determined sarcopaenia characterised by severe muscle wasting has been shown to be an indicator of adverse outcomes in several populations6,7,8. An abdominal computed tomography (CT) scan-derived skeletal muscle mass index is an accurate method to measure it8. Current data regarding the prevalence and outcomes of sarcopaenia in TAVR are sparse. We designed this study to compare a number of short-term outcomes and overall mortality in sarcopaenic and non-sarcopaenic patients after TAVR.
Methods
STUDY POPULATION
Patients with severe AS who underwent TAVR from February 2012 to June 2016 at the Mayo Clinic, Rochester, MN, USA, were retrospectively reviewed. All patients underwent TAVR using either the SAPIEN (SAPIEN, SAPIEN XT, or SAPIEN 3; Edwards Lifesciences, Irvine, CA, USA) or the CoreValve® (CoreValve® or Evolut™ R; Medtronic, Minneapolis, MN, USA) prosthesis. The study protocol was approved by the Mayo Clinic Institutional Review Board.
CLINICAL AND LABORATORY MEASUREMENTS
The age, sex, diabetes mellitus status, and New York Heart Association (NYHA) heart failure of participants were retrospectively reviewed from the valve registry database at the Mayo Clinic. History of moderate to severe pulmonary disease, atrial fibrillation, stroke, peripheral arterial disease, myocardial infarction, percutaneous coronary angioplasty, and coronary artery bypass grafting was also obtained from the database.
Computed tomography (CT) scan data were used to obtain ascending aorta and aortic valve calcium content. Baseline echocardiography was performed in all patients before the TAVR procedure according to the guidelines of the American Society of Echocardiography9. The biplane Simpson method was used to measure left ventricular ejection fraction. Stroke volume was determined using the Doppler method by simplified continuity equation. Aortic valve area and gradient were measured by simplified continuity and Bernoulli equations, respectively. Valvular regurgitation was graded according to the established guidelines10.
MEASUREMENT OF ABDOMINAL MUSCLE MASS
An abdominal CT scan at the level of the L3 vertebra was used to measure abdominal muscle mass. The recently revised European consensus on the definition and diagnosis of sarcopaenia states that a CT scan is the gold standard for non-invasive measurement of muscle mass. Abdominal muscle mass measured by CT scan at the level of the L3 lumbar vertebra is a good indicator of total body muscle mass and its lower values are associated with adverse outcomes in different patient populations8. Erector spinae, quadratus lumborum, psoas major and minor, internal, external, and transverse abdominis muscles are involved in the measurement of muscle mass in this technique (Figure 1). Muscle density was defined as densities from –30 to 150 Hounsfield units (HU) and skeletal muscle index was calculated by normalising skeletal muscle area by height (m²) and reported as cm²/m². We used our previously validated semi-automated software for body composition analysis11,12.
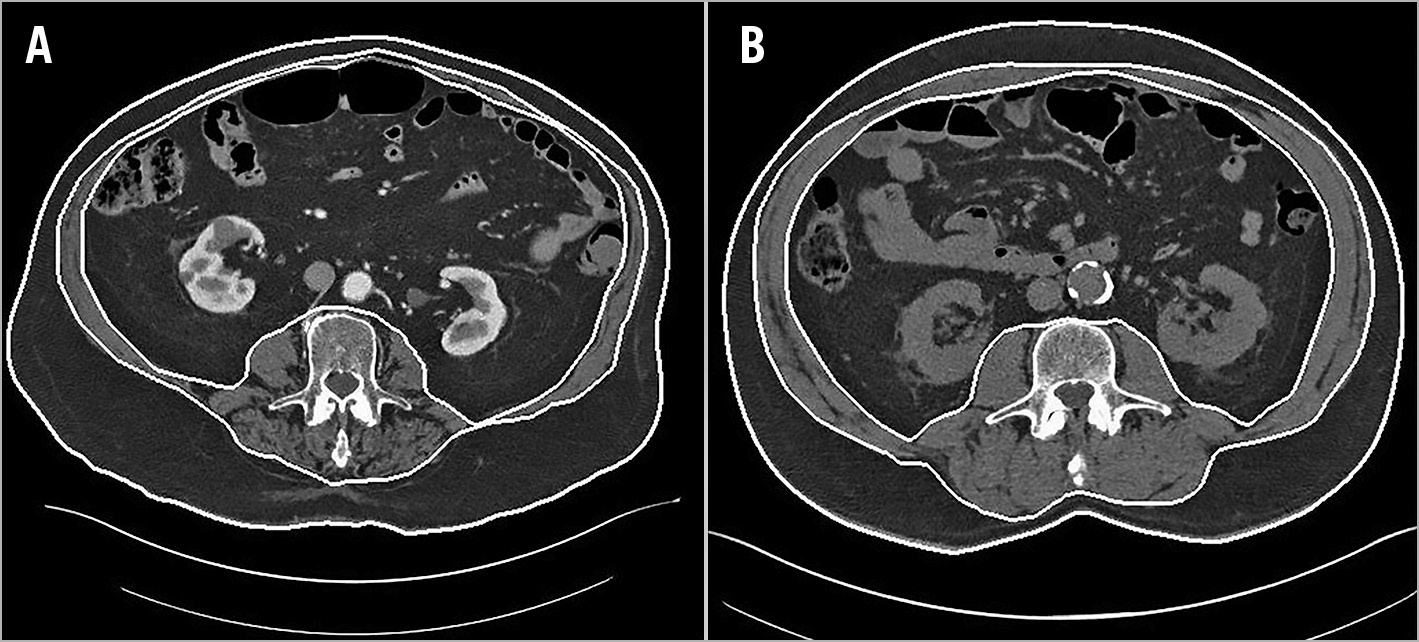
Figure 1. CT scans of a sarcopaenic patient and a non-sarcopaenic patient. A) Sarcopaenic patient. B) Non-sarcopaenic patient.
DEFINITIONS
Glomerular filtration rate was calculated using the Modification of Diet in Renal Disease study equation formula. Severe chronic kidney disease was defined as stage 4 or 5 chronic renal failure (glomerular filtration rate <30 mL/min/1.73 m²)13. Left ventricular hypertrophy was defined as echocardiography-derived left ventricular mass index higher than 115 g/m² for men and more than 95 g/m² for women14. The Society of Thoracic Surgeons (STS) risk score was calculated for each patient in the pre-TAVR clinic using previously published guidelines (2008) and reported from our valve registry database. Sarcopaenia was defined as skeletal muscle index values <55.4 cm²/m² in males and <38.9 cm²/m² in females. These gender-specific cut-off values are previously published values for cancer patients12,15 and were used in a previously published TAVR study16.
OUTCOMES
All-cause mortality was the primary outcome and was obtained by reviewing the clinical charts. Length of hospital stay, defined as the time from the TAVR procedure to discharge, and intensive care unit (ICU) stay were reported in days. Valve Academic Research Consortium (VARC)-2 definitions3 were used for 30-day adverse outcomes. VARC-2 life-threatening or disabling bleeding and major bleeding, access-site major and minor vascular complications, myocardial infarction, transient ischaemic attack (TIA)/stroke, and conduction abnormalities needing pacemaker or intra-cardiac defibrillators as well as the need for haemodialysis were studied in both groups. Pre-discharge paravalvular leak was determined by an experienced echocardiographer as per transvalvular transcatheter registry guidelines3. We considered more than mild paravalvular leak as a significant outcome.
STATISTICAL ANALYSIS
The baseline characteristics of the participants are presented as mean±standard deviation for continuous variables and number plus percent for dichotomous variables. The study population was divided into sarcopaenic and non-sarcopaenic groups. Dichotomous variables were compared using the χ2 or Fisher’s exact test, normally distributed continuous variables were compared by t-test, and non-normally distributed continuous variables were compared with the Mann-Whitney U test.
Kaplan-Meier analysis and the log-rank test were used to determine overall survival in both groups. Stepwise Cox regression analysis was used to assess determinants of overall mortality. First, we performed univariable analysis to identify possible predictors of overall mortality. Second, variables with p-values <0.2 in the univariable analysis as well as age and sex were included in the multivariable analysis. Next, we eliminated variables with p-values >0.1, one by one, from our multivariable model. Finally, after excluding all the variables with p-values >0.1, we added the other variables that initially had p-values >0.2 in the univariable analysis and were not included in the multivariable model at the first step. We added them one by one and kept them in the model if they had p-values <0.1 to achieve our final model. To evaluate the association of sarcopaenia with mortality in a matched cohort, we performed inverse propensity score weighting analysis17. Baseline variables as well as comorbid conditions including age, sex, body mass index, severe chronic kidney disease, history of myocardial infarction, peripheral arterial disease, moderate or severe chronic lung disease, atrial fibrillation or flutter, left ventricular ejection fraction, tricuspid regurgitation, porcelain aorta, and plasma haemoglobin level were included in the inverse propensity score weighting analysis. These variables were included in the model either because they have been associated with mortality in our study or previous studies or because they were significantly different at baseline between sarcopaenic and non-sarcopaenic patients in our study. We used JMP 13 (SAS Institute Inc., Cary, NC, USA) and R, version 3.3 (R Foundation for Statistical Computing, Vienna, Austria) for data analysis and considered two-sided p-values less than 0.05 as statistically significant.
Results
BASELINE CHARACTERISTICS OF THE STUDY POPULATION
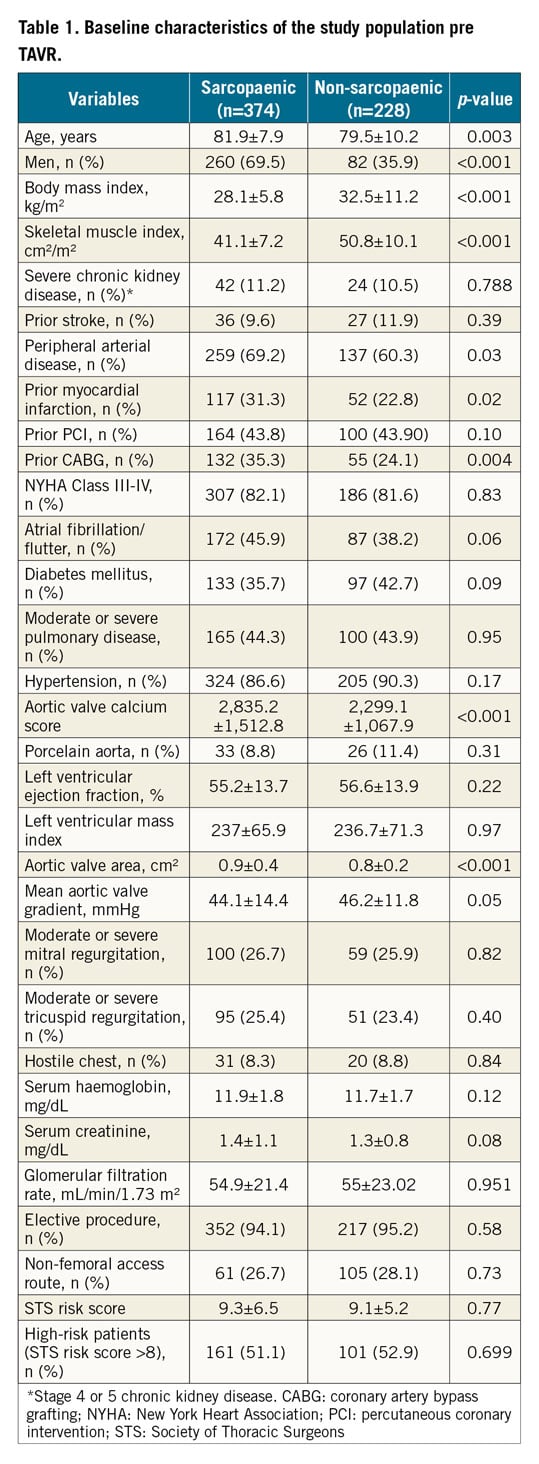
Six hundred and two (602) patients underwent TAVR and were followed up for a median of 1.5 years. The mean age±SD of the study population was 80.9±8.9 years and 284 (56.8%) of the patients were male. Sarcopaenia was prevalent in our study population (374 patients, 62.1%). Two hundred and sixty (260; 69.5%) out of 374 sarcopaenic patients and 82 (35.9%) out of 228 non-sarcopaenic patients were male. Sarcopaenic patients were slightly older (81.9±7.9 years vs 79.5±10.2 years, p<0.001) and had lower body mass index (28.1±5.8 kg/m2 vs 32.5±11.2 kg/m2, p<0.001). Mean±SD skeletal muscle index was 41.1±7.2 cm²/m² in sarcopaenic and 50.8±10.1 cm²/m² in non-sarcopaenic individuals (p<0.001). Most other variables including STS risk score as well as valve success were not different between the two groups (Table 1).
CLINICAL OUTCOMES
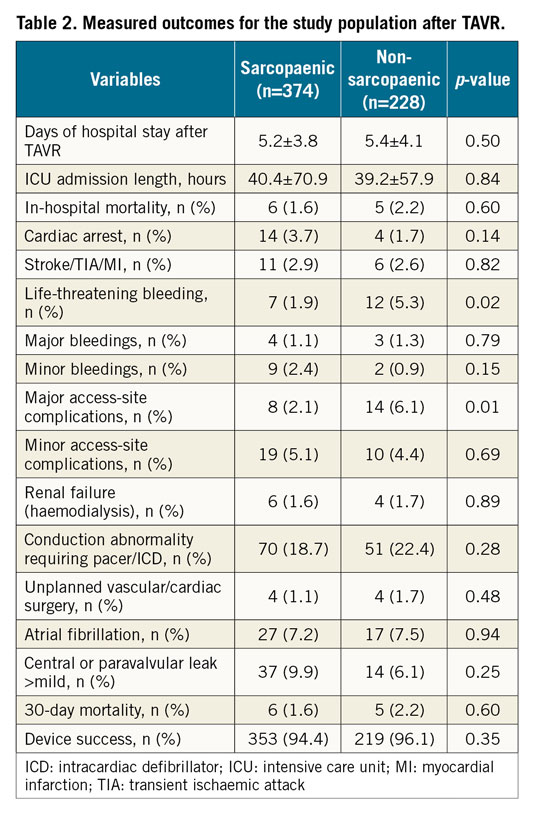
Measured 30-day adverse outcomes in our study are shown in Table 2. Life-threatening bleeding (5.3% vs 1.9%, p=0.02) and major vascular complications (6.1% vs 2.1%, p=0.02) were more prevalent in non-sarcopaenic patients (Table 2). The majority of vascular complications occurred with the femoral access route (18 out of 22 cases). There was no significant correlation between access-site type (femoral and non-femoral) and access-site complications assessed by χ2 test (p=0.273). The other 30-day adverse outcomes were not significantly different between the two groups.
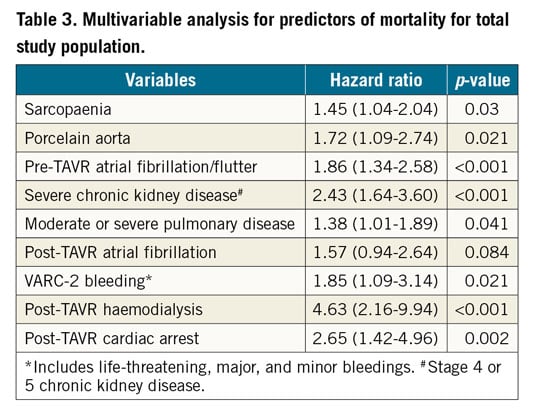
One hundred and twelve deaths (112; 29.9%) occurred in sarcopaenic and 53 deaths (23.3%) occurred in non-sarcopaenic patients during follow-up. Sarcopaenic patients had significantly worse survival than non-sarcopaenic patients during four-year follow-up (log-rank p=0.022) (Figure 2). In the final multivariable model for predictors of mortality, sarcopaenia (HR=1.45, p=0.03) remained independently associated with overall mortality (Table 3).
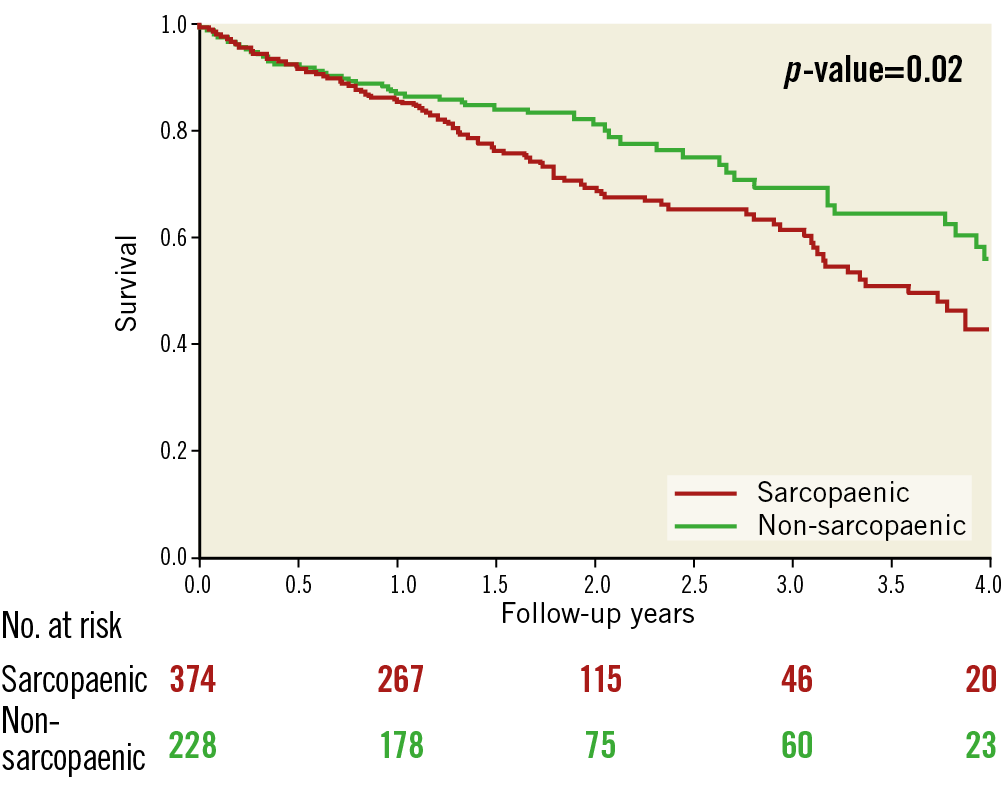
Figure 2. Survival at four-year follow-up.
SEX-STRATIFIED CLINICAL OUTCOMES
We observed no major difference regarding baseline characteristics and short-term study outcomes when the analyses were performed for men and women separately (Supplementary Table 1, Supplementary Table 2).
INVERSE PROPENSITY SCORE WEIGHTING ANALYSIS
Sarcopaenia remained significantly associated with mortality in the inverse propensity score-matched analysis (HR=1.4, p=0.048) as well as following adjustment for post-TAVR predictors of mortality in the model (HR=1.49, p=0.019) (Supplementary Table 3).
Discussion
In the current study, we demonstrated that 1) 2/3 of patients undergoing TAVR have sarcopaenia with higher predominance in men, and 2) patients with sarcopaenia have worse long-term survival post TAVR compared to non-sarcopaenic patients without increased vulnerability to TAVR complications. Our study shows that TAVR is a safe procedure in sarcopaenic patients and suggests the potential value of systematic evaluation of sarcopaenia prior to TAVR to decide the best treatment pathways or potentially to consider special rehabilitation and nutritional programmes in these vulnerable patients.
Similar to AS, sarcopaenia is a biological process associated with ageing. There is a progressive decline in muscle mass and function with ageing which starts in the third decade of life but is not usually prominent until the fifth decade18. After the age of 60, there is an annual decrease of up to 3 percent in muscle mass. Decreased muscle mass contributes to functional impairment directly by decreased muscle function and strength and indirectly by accelerating osteoporosis and the subsequent increase in the rate of fractures. This leads to decreased quality of life and earlier death5,18. Patients with radiologically determined sarcopaenia have poor outcomes such as overall mortality in several patient populations6,7,12. Sarcopaenic patients are specifically vulnerable to major stressors such as major surgeries and perioperative complications6,19. However, in our study, sarcopaenic patients did not have increased vulnerability to perioperative complications and morbidity. Interestingly, patients without sarcopaenia were more likely to have significant VARC-2 life-threatening bleeding and major vascular complications which were not correlated with the access-site type for TAVR. These findings could be related to a higher prevalence of obesity in non-sarcopaenic patients which could potentially increase the rates of access site-related complications as observed in this study. Overall, these findings support the safety of TAVR in patients with sarcopaenia.
Two previous studies have shown that sarcopaenia and low skeletal muscle mass index were associated with increased length of hospital stay20 and overall mortality16 in patients undergoing TAVR. Similar to our study, both studies showed that sarcopaenia was highly prevalent in patients undergoing TAVR. Dahya et al showed that skeletal muscle mass index is inversely associated with length of hospital stay in 104 patients following TAVR20. Our study extends this previous observation and demonstrates that there is no significant difference between sarcopaenic and non-sarcopaenic patients regarding length of hospital stay. Although our sample size was larger than the mentioned study, skeletal muscle mass index was not associated with length of hospital stay (Supplementary Figure 1). Similar to our study, Mok et al showed that sarcopaenia is associated with two-year mortality following TAVR. Pre-TAVR atrial fibrillation, chronic lung disease, renal failure, and male sex were also associated with mortality in their study population. However, short-term TAVR complications in sarcopaenic and non-sarcopaenic individuals were not reported in this study16.
Sarcopaenia remained an independent predictor of mortality in our study in the multivariable analysis of predictors of mortality. Other baseline variables such as porcelain aorta, pre-TAVR atrial fibrillation, chronic kidney disease, and moderate to severe pulmonary disease were associated with mortality in our study. Among TAVR complications, post-TAVR renal failure requiring haemodialysis, VARC-2 defined bleedings, and post-TAVR cardiac arrest were associated with mortality in our study. Our findings are in line with the previously published studies2,3,21,22,23.
Although assessment of frailty is a comprehensive evaluation to predict adverse outcomes, it is relatively subjective and has potential variability in the results. In addition, due to the high prevalence of comorbidities in TAVR populations, several patients might not be able to do some parts of the frailty assessment such as the walk test. Compared to frailty, diagnosing sarcopaenia using a CT scan is an objective measure which can be available in all TAVR centres. A pre-TAVR abdominal CT scan is almost uniformly carried out in TAVR centres and thus is available without any extra cost. Assessment of sarcopaenia using a computer program can be done in a short time and at low cost. Therefore, sarcopaenia is worth evaluating to see whether it can be incorporated in a standard hospital performing TAVR procedures. Furthermore, diagnosing sarcopaenia can identify patients who may benefit from additional nutritional support, treatment of co-existing morbidity, cardiac rehabilitation, or exercise programmes. It has been shown that protein and amino acid supplements combined with resistance training exercises can increase muscle mass and function. Vitamin D supplementation, hormone replacement therapy in post-menopausal women, and medications such as Ghrelin secretagogues have also been shown to have a beneficial effect in increasing muscle mass and function5. However, whether such interventions can lead to improvement in overall clinical outcomes of sarcopaenic patients will need further investigation.
As with any other new concept in medicine, the definition of sarcopaenia is still evolving. While a combination of muscle mass, function, and strength is involved in the definition of sarcopaenia, radiologically defined sarcopaenia is an objective definition which relies on the measurement of the muscle mass using imaging techniques. The recently published updated European consensus on definition and diagnosis of sarcopaenia proposes that magnetic resonance imaging (MRI) and CT scan should be the gold standard methods for non-invasive measurement of body muscle mass8. Specifically, measurement of muscle mass using a CT scan at the lumbar L3 vertebra is significantly correlated with the total body muscle mass8. It can objectively detect sarcopaenia at the early stages, and low muscle mass diagnosed by this method has been shown to be correlated with adverse outcomes in different patient populations. However, accurate cut-off values to diagnose sarcopaenia using this technique are still being developed8. In the current study, we used the cut-off values that were previously defined and used for cancer patients since there have been no previously validated cut-offs in patients who undergo TAVR15. Although these cut-off values are well known and frequently used in clinical research, it is important to note that they were defined for cancer patients who are younger compared to elderly patients who undergo TAVR. Interestingly, similar to our study, Mok et al have used an abdominal CT scan at the level of L3 to assess muscle mass index. They used the same cut-offs as ours to define sarcopaenia in men and women and found that it was associated with two-year mortality in their patients16. It is reassuring that the same technique and cut-offs to define sarcopaenia in Canadian patients had a reproducible outcome in a U.S. patient population with a relatively longer follow-up time. These combined findings add more weight to using an abdominal CT scan to assess muscle mass in TAVR patients in North America.
Limitations
Our study has some limitations. Although we showed that TAVR is a safe procedure in sarcopaenic patients, we did not identify the cause of mortality. Therefore, we could not identify whether this observed higher mortality was due to cardiac causes and valve failure or other comorbidities which are not necessarily correlated with the TAVR procedure. In addition, frailty data, including serum albumin level, were not uniformly available for our study population. Therefore, we were not able to assess whether sarcopaenia is an independent predictor of mortality were the model to have included frailty measures as well. Future studies can assess whether sarcopaenia has added value to frailty to predict adverse events following TAVR or whether treatment of sarcopaenia can improve long-term survival following TAVR.
Conclusions
In conclusion, sarcopaenia is common in patients undergoing TAVR. Sarcopaenic patients have comparable in-hospital outcomes to non-sarcopaenic patients. However, sarcopaenia was an independent risk factor for overall mortality. The current study supports the safety of TAVR in patients with sarcopaenia and the major effect of sarcopaenia on long-term mortality, arguing for its potential value in the systematic evaluation of this highly comorbid population in order to decide the best treatment pathways or potentially to consider special rehabilitation and nutritional programmes in these vulnerable patients.
|
Impact on daily practice Transcatheter aortic valve replacement (TAVR) is commonly performed in elderly frail or high-intermediate surgical risk patients with severe aortic valve stenosis. In this study we found that sarcopaenia is prevalent in two thirds of TAVR patients and is independently associated with increased mortality, although sarcopaenic patients have comparable in-hospital outcomes, arguing for the safety of TAVR in this high-risk population. This study supports further investigations on best treatment pathways (e.g., TAVR versus surgical aortic valve replacement) in this unique patient population. |
Funding
This publication was made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Conflict of interest statement
The authors have no conflicts of interest to declare.

