Abstract
Background: Morbidly obese (MO) patients are increasingly undergoing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) for severe aortic stenosis (AS). However, the best therapeutic strategy for these patients remains a matter for debate.
Aims: Our aim was to compare the periprocedural and mid-term outcomes in MO patients undergoing TAVR versus SAVR.
Methods: A multicentre retrospective study including consecutive MO patients (body mass index ≥40 kg/m2, or ≥35 kg/m2 with obesity-related comorbidities) from 18 centres undergoing either TAVR (n=860) or biological SAVR (n=696) for severe AS was performed. Propensity score matching resulted in 362 pairs.
Results: After matching, periprocedural complications, including blood transfusion (14.1% versus 48.1%; p<0.001), stage 2-3 acute kidney injury (3.99% versus 10.1%; p=0.002), hospital-acquired pneumonia (1.7% versus 5.8%; p=0.005) and access site infection (1.5% versus 5.5%; p=0.013), were more common in the SAVR group, as was moderate to severe patient-prosthesis mismatch (PPM; 9.9% versus 39.4%; p<0.001). TAVR patients more frequently required permanent pacemaker implantation (14.4% versus 5.6%; p<0.001) and had higher rates of ≥moderate residual aortic regurgitation (3.3% versus 0%; p=0.001). SAVR was an independent predictor of moderate to severe PPM (hazard ratio [HR] 1.80, 95% confidence interval [CI]: 1.25-2.59; p=0.002), while TAVR was not. In-hospital mortality was not different between groups (3.9% for TAVR versus 6.1% for SAVR; p=0.171). Two-year outcomes (including all-cause and cardiovascular mortality, and readmissions) were similar in both groups (log-rank p>0.05 for all comparisons). Predictors of all-cause 2-year mortality differed between the groups; moderate to severe PPM was a predictor following SAVR (HR 1.78, 95% CI: 1.10-2.88; p=0.018) but not following TAVR (p=0.737).
Conclusions: SAVR and TAVR offer similar mid-term outcomes in MO patients with severe AS, however, TAVR offers some advantages in terms of periprocedural morbidity.
Introduction
Worldwide, the obesity epidemic continues to grow across low-, middle- and high-income countries. The World Health Organization (WHO) has reported a tripling in the prevalence of obesity between 1975 and 20161. In the United States, it is estimated that by 2030, 50% of the population will be obese, with 25% having severe obesity (body mass index [BMI] ≥ 35 kg/m2)2. Together with this growing obesity problem, our population is ageing, with increased rates of age-related degenerative diseases such as aortic stenosis (AS). Treatment of such diseases in obese patients is increasing in frequency and presents a significant challenge. Surgical aortic valve replacement (SAVR) in obese patients can result in a number of periprocedural difficulties, including problems with ventilation during anaesthesia3, respiratory infections4, impaired wound and sternotomy healing, access site and sternal infections567, and prolonged hospital stays7. Transcatheter aortic valve replacement (TAVR) has rapidly evolved to become a viable alternative treatment for symptomatic severe AS with at least comparable, and in some studies superior, outcomes to SAVR, across a wide spectrum of low- to high-risk patients8. Among these trials, however, morbidly obese (MO) patients are underrepresented, and extrapolating these findings to MO populations may not be fully supported by evidence. A recent multicentre registry showed comparable mid-term outcomes in MO patients undergoing TAVR versus their non-obese counterparts, although major vascular complications were more common in the MO group9. This suggests a significant potential benefit for TAVR in this population, circumventing many of the periprocedural difficulties associated with SAVR in MO patients. Furthermore, TAVR, in comparison to SAVR, is associated with less prosthesis-patient mismatch (PPM), a commonly encountered phenomenon in MO patients. As PPM has been associated with poorer outcomes in SAVR populations101112, procedures such as TAVR, with less PPM, may be of significant value in this population. Nevertheless, outcome data directly comparing TAVR to SAVR in this group are scarce and limited to “moderately obese” (BMI ~30-35 kg/m2) patients treated with early-generation TAVR valves13. We, therefore, aimed to compare periprocedural and mid-term outcomes in MO patients undergoing TAVR or SAVR for symptomatic severe AS.
Methods
This was a retrospective multicentre, observational study involving 18 tertiary care centres in Europe and North America, including consecutive MO patients undergoing TAVR between 2008 and 2019. In addition, 8 centres provided data on consecutive MO patients undergoing SAVR, as a comparator group. The decision to perform either TAVR or SAVR was made at each individual centre, according to current guidelines and local protocols. All commercially available TAVR and biological SAVR valves were included. Patients with valve-in-valve procedures were excluded. Patients who underwent mechanical aortic valve implantation or concomitant replacement of other cardiac valves were also excluded, as were those requiring concomitant repair of the thoracic aorta. Patients undergoing SAVR or TAVR with concomitant coronary revascularisation were included. Both TAVR and SAVR were performed, as previously described, using manufacturers’ recommendations for deployment in the case of TAVR1415. Patients undergoing TAVR by all access routes were included, along with those undergoing SAVR by midline sternotomy and mini-sternotomy. Other procedure-related aspects were at the operators´ discretion. All patients signed informed consent for the procedure, and the study was performed in accordance with the institutional review board of the participating centres.
BMI was calculated as: weight in kg/height in metres squared (m²). Morbid obesity was defined as BMI ≥40 kg/m2, or ≥35 kg/m2 with obesity-related comorbidities1617. All data, including baseline, periprocedural and clinical follow-up data, were prospectively collected in a dedicated database at each participating centre, and statistical analysis was performed by the coordinating centre. Periprocedural events were defined using the Valve Academic Research Consortium-2 (VARC-2) criteria18.
PPM was defined using the VARC-3 criteria19. For this calculation, previously defined predicted effective orifice area (EOA) for each valve type and size were used2021 and indexed (iEOA) to body surface area (BSA), calculated from the Dubois formula. Predicted EOA was chosen due to its closer association with transprosthetic gradients22. BMI-specific cut-offs were used to determine the presence of PPM; as such, PPM was considered to be: none, if iEOA was >0.70 cm2/m2; moderate, if iEOA was 0.56-0.70 cm2/m2; and severe, if iEOA was ≤0.55 cm2/m2192123. Clinical follow-up was at 30 days, 6 months, and yearly thereafter. Mid-term outcomes were assessed at 24 months.
The primary outcome was 2-year all-cause mortality. Secondary outcomes included in-hospital mortality, periprocedural complications, valve performance and patient-prosthesis mismatch.
Statistical analysis
Categorical variables were expressed as numbers and percentages, while continuous variables were expressed as mean and standard deviation (SD), or median and interquartile range (IQR, 25th-75th percentile), according to their distribution. Normality was assessed using the Kolmogorov-Smirnov test. For the comparison of study groups (TAVR versus SAVR), qualitative variables were analysed using the chi-squared or the Fisher´s exact test, and differences in continuous variables were analysed using a 2-sided Student´s t-test or Kruskall-Wallis test for the unmatched comparison. A non-parsimonious propensity score-matched analysis was performed between the 2 groups. A propensity score was estimated using a logistic regression model. The treatment group (TAVR or SAVR) was the dependent variable; independent variables were those baseline characteristics found to have statistically significant differences between TAVR and SAVR groups, and other variables considered to be clinically relevant. The final variables included in the propensity matching were: age, sex, BMI, pre-existing coronary artery disease (CAD) , prior coronary artery bypass grafting (CABG), estimated glomerular filtration rate (eGFR), risk score, pre-existing peripheral vascular disease, chronic obstructive pulmonary disease (COPD), and atrial fibrillation. The Society of Thoracic Surgeons (STS) score or EuroSCORE II were used as risk scores. Risk categories were defined as: low risk (score <4), or intermediate to high risk (score ≥4). A propensity score-matched cohort was then created with a 1:1 ratio of TAVR and SAVR patients using a “nearest neighbour” match without replacement. A caliper width of <0.1 x the SD of the logistic score was applied. The appropriateness of the matching was assessed in several ways: first, smoothed kernel density plots of the logistic score were computed in order to visually assess the balance between groups before and after matching (Supplementary Figure 1). Then, standardised mean differences (SMD) were calculated for all covariates (both those included and not included in the logistic score calculation) in order to assess for potential imbalances between TAVR and SAVR cohorts. Comparison of continuous and categorical variables between the matched groups were as previously stated for unmatched groups. Freedom from mortality and readmission curves were calculated using the Kaplan-Meier method and compared using the stratified log-rank test in the matched cohorts24. Post-match adjustment for variables found to have significant imbalances by variance ratio after matching was also performed as an additional calculation, using multivariable Cox regression. To reflect more contemporary practices, a second analysis was performed restricting the population to only those patients who underwent TAVR or SAVR after 2014. Propensity score matching in this more contemporary population was performed as previously outlined.
Predictors of 2-year all-cause mortality were also assessed separately for the TAVR and SAVR groups using Cox regression analysis. Variables with a p-value of <0.1 on univariable analysis were entered into the multivariable analysis, and those with resulting p-values <0.05 were considered statistically significant. Logistic regression analysis was used to assess predictors of PPM in the overall cohort in a similar fashion. All data were analysed with Stata 15.1 (StataCorp).
Results
Patient population
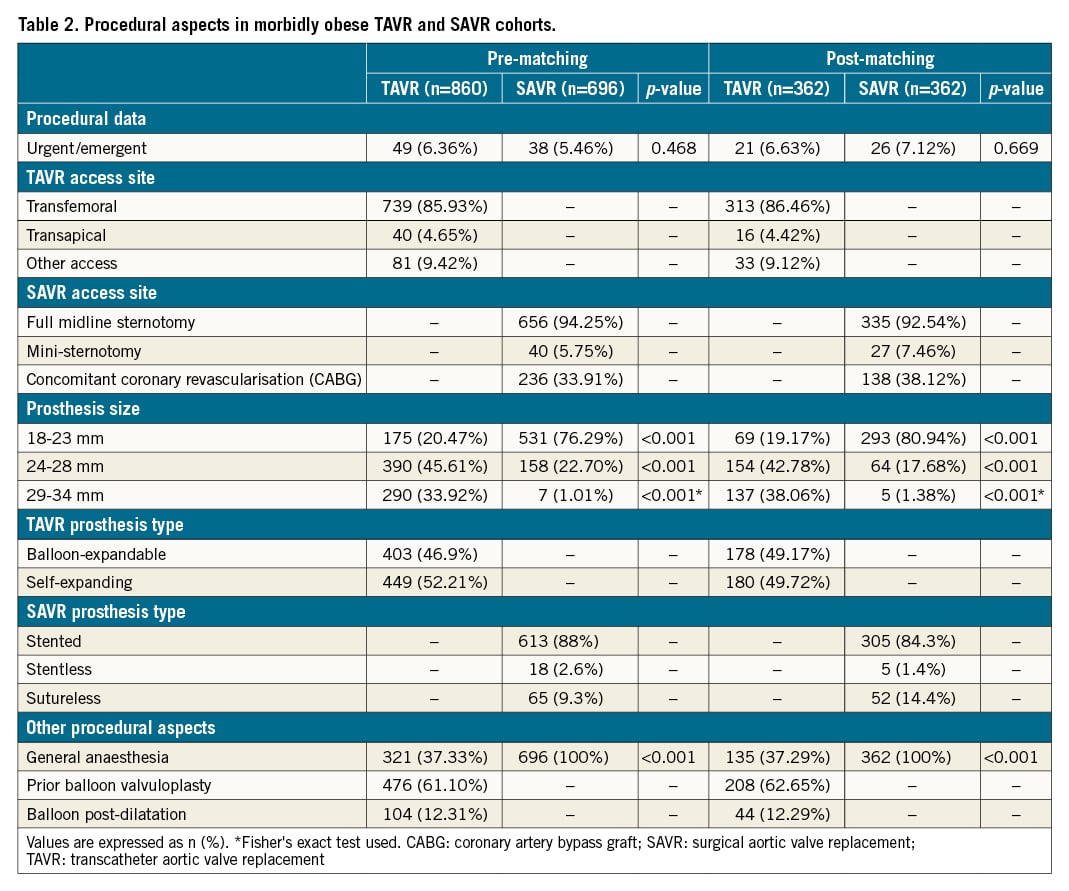
A total of 1,556 consecutive MO patients were included: 860 in the TAVR group, and 696 in the SAVR group. Baseline characteristics of the overall population are summarised in Table 1. A number of baseline characteristics differed significantly between the groups. TAVR patients were older (77 versus 71 year;; p<0.001), more commonly female (67.3 versus 52.6; p<0.001) and more frequently had other significant comorbidities, including higher rates of hypertension, previous CAD, COPD and lower baseline eGFR (p<0.05 for all variables). Consequently, surgical risk scores were higher in the TAVR group when compared to SAVR. Procedural data for both groups are summarised in Table 2. The transfemoral approach was used in 86% of the TAVR cohort with midline sternotomy access being used in 94.2% of the SAVR population. Smaller valve sizes (18-23 mm) were more frequently used in the SAVR group (20.5% in TAVR versus 79.3%; p<0.001). The type of bioprosthesis used is outlined in Table 2 and Supplementary Table 1.
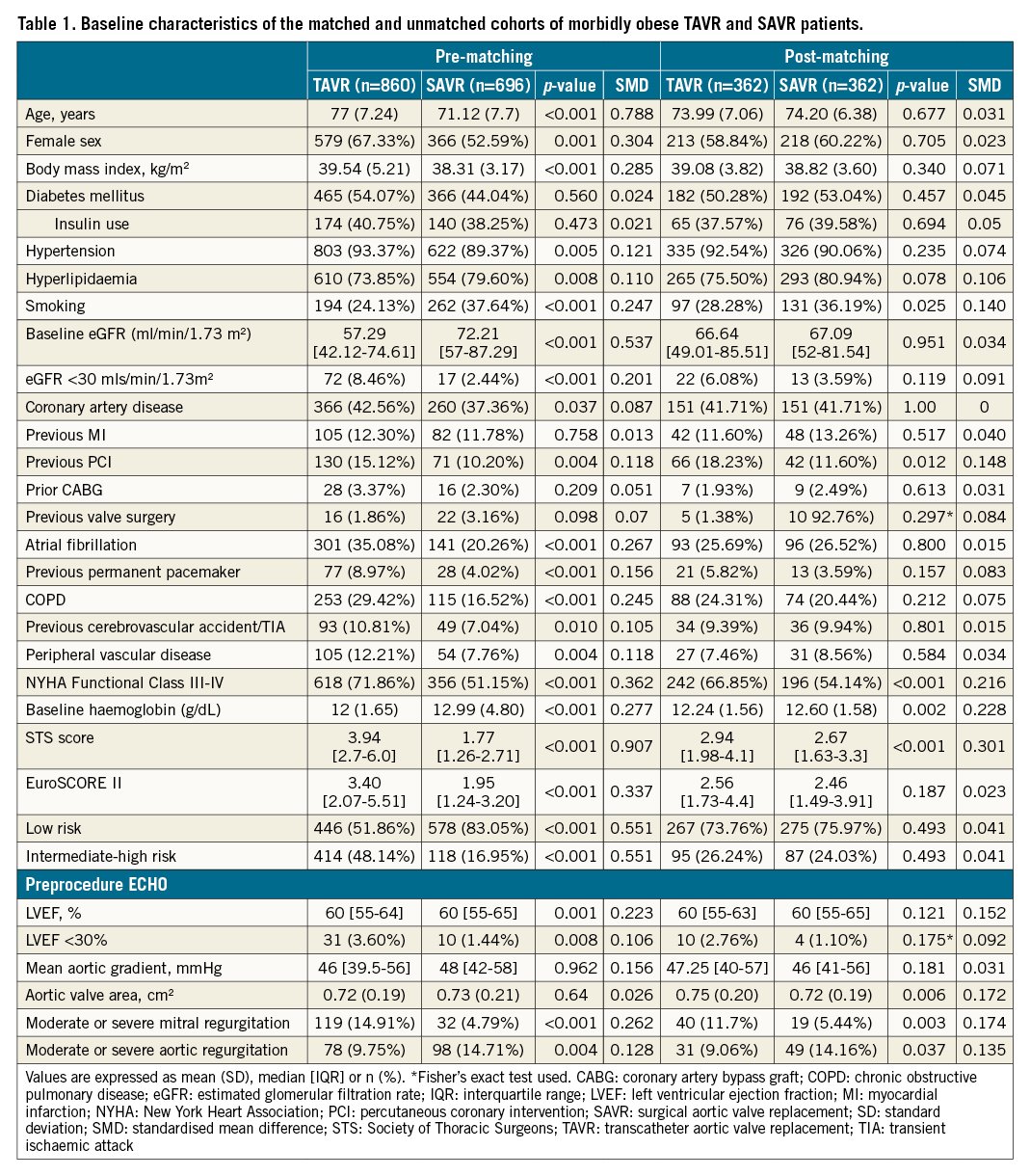
Matched cohort
Propensity score matching resulted in 362 matched pairs. Close matching was observed as depicted in Supplementary Figure 1, although some differences remained, with SMD being >0.10 for some variables. The TAVR group continued to have higher overall surgical risk scores (Table 1). However, both cohorts were predominantly defined as low risk. Additionally, the TAVR group had higher rates of multivalvular disease, with more patients having moderate to severe mitral regurgitation (11.7% versus 5.4%; p=0.002) at baseline.
In-hospital outcomes
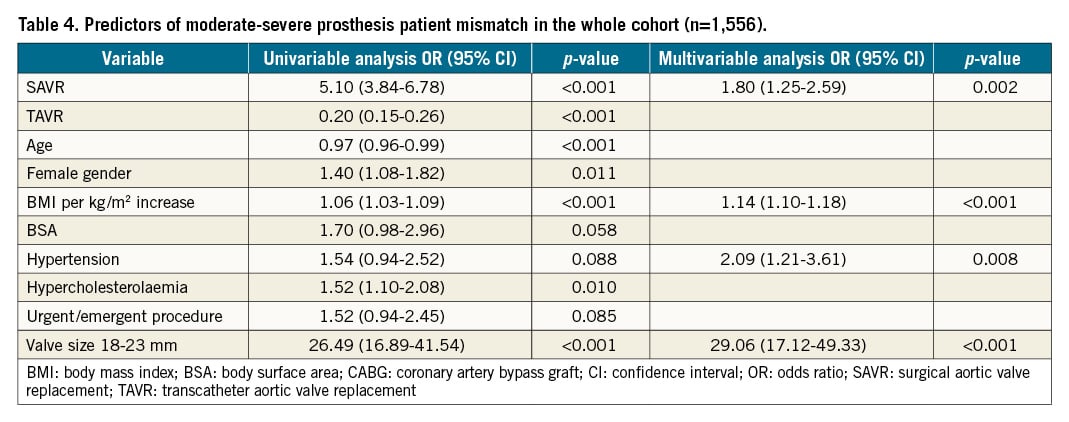
Table 3 summarises the in-hospital outcomes for both the matched and unmatched populations. After matching, in-hospital mortality was numerically more common in the SAVR group (3.9% versus 6.1%, for TAVR and SAVR, respectively), but this did not reach statistical significance (p=0.171). No differences in vascular complications, or life-threatening or major bleeding were found between groups. However, the SAVR group required significantly more blood transfusions (14.1% versus 48.1%; p<0.001). Stage 2-3 acute kidney injury (AKI) was more common in the SAVR group (3.99% versus 10.1%; p=0.002), as was hospital-acquired pneumonia (1.7% versus 5.8%; p=0.005), and access site infection (1.5% versus 5.5%; p=0.013), while TAVR patients more commonly required permanent pacemaker implantation during the index admission (14.4% versus 5.6%, for TAVR and SAVR, respectively; p<0.001) (Central illustration). Regarding valve performance, residual ≥moderate aortic regurgitation was higher following TAVR (3.3% versus 0% in SAVR; p=0.001). Higher post-procedural mean aortic valve gradients (10.5 versus 15 mmHg; p<0.001), with higher rates of mean gradient >20 mmHg (8% versus 26.3%; p<0.001) and increased rates of moderate to severe PPM (9.9% versus 39.4%; p<0.001) were found in the SAVR group. Predictors of PPM in the overall cohort included SAVR (HR 1.80, 95% CI: 1.25-2.59; p=0.002), elevated BMI, hypertension and use of smaller prosthesis size (18-23 mm) (Table 4). Overall, SAVR patients had longer inpatient admissions than TAVR patients (median 5 versus 9 days for SAVR; p<0.001).
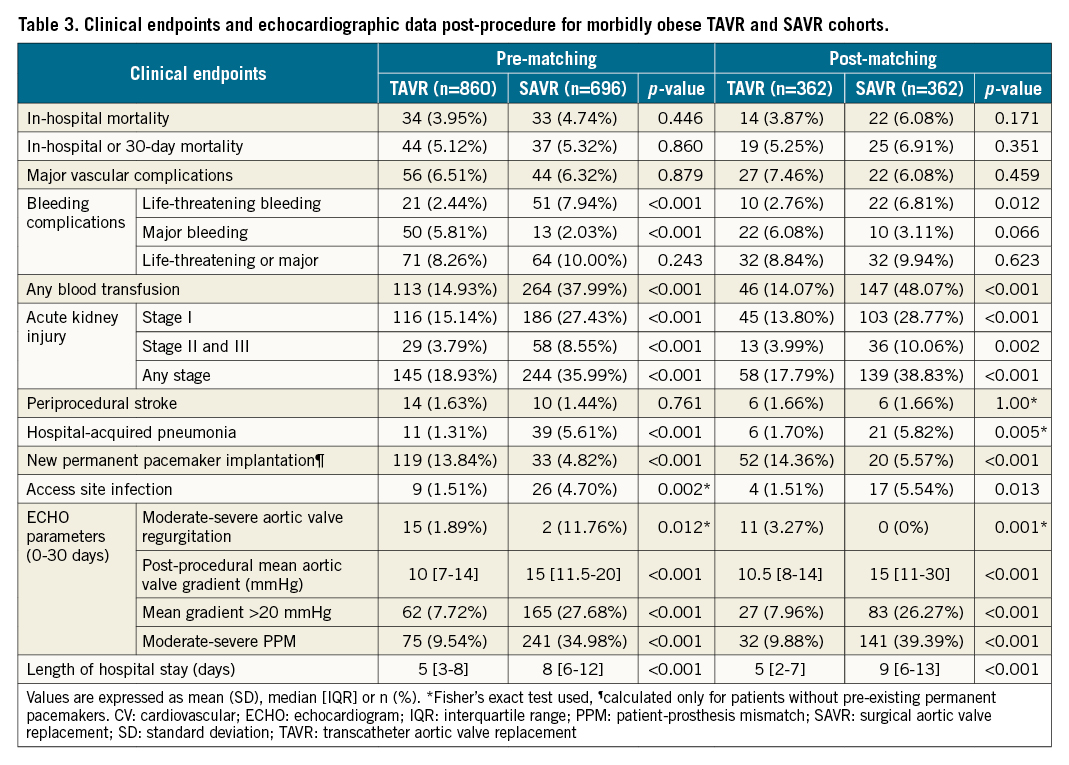
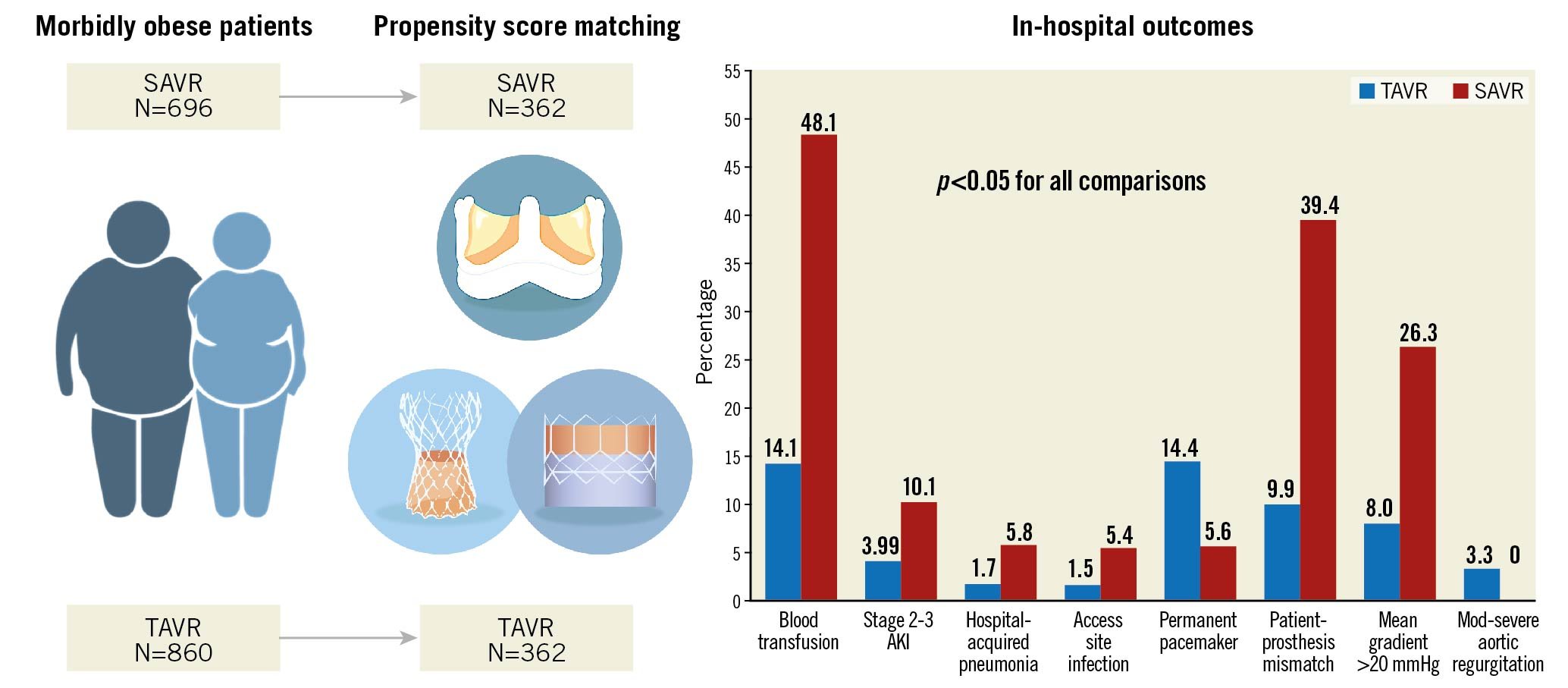
Central illustration: In-hospital outcomes following propensity score matching of morbidly obese patients undergoing TAVR versus SAVR. AKI: acute kidney injury; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement
Mid-term outcomes
The median follow-up was 33.2 months (IQR 12.9-61.6). Kaplan-Meier graphs depicting mid-term outcomes for the matched cohort are shown in Figure 1A-Figure 1D. At 2 years, the primary outcome of freedom from all-cause mortality was similar for both TAVR and SAVR groups (84.1% versus 85.8%, log-rank p=0.651), as was cardiovascular (CV) mortality (89.9% versus 89%, log-rank p=0.686, for TAVR and SAVR, respectively). Similarly, all-cause and CV readmissions were not different between matched TAVR and SAVR groups. Kaplan-Meier curves for the unmatched cohort are shown in Supplementary Figure 2. After adjusting mid-term outcomes for those variables whose variance ratio was different between groups (eGFR, left ventricular ejection fraction [LVEF] and risk score), no differences in all-cause and CV mortality were found between groups. However, all-cause readmissions were higher in the SAVR group (HR 1.45, 95% CI: 1.04-2.02; p=0.029) (Supplementary Table 2).
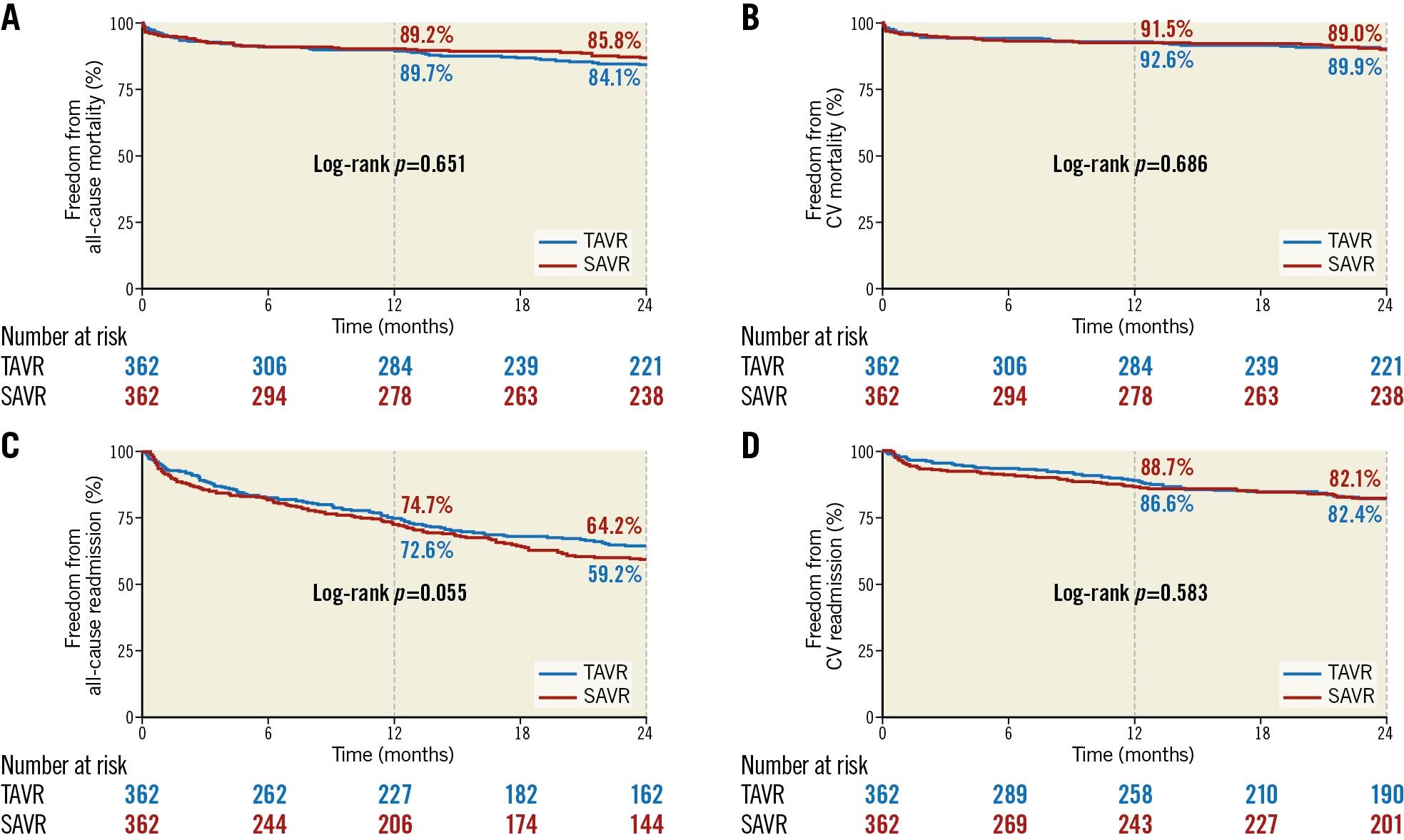
Figure 1. Kaplan-Meier graph demonstrating 2-year all-cause (A) and cardiovascular (B) mortality and 2-year all-cause (C) and cardiovascular (D) readmission in the propensity score-matched analysis for morbidly obese TAVR and SAVR groups. CV: cardiovascular; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement
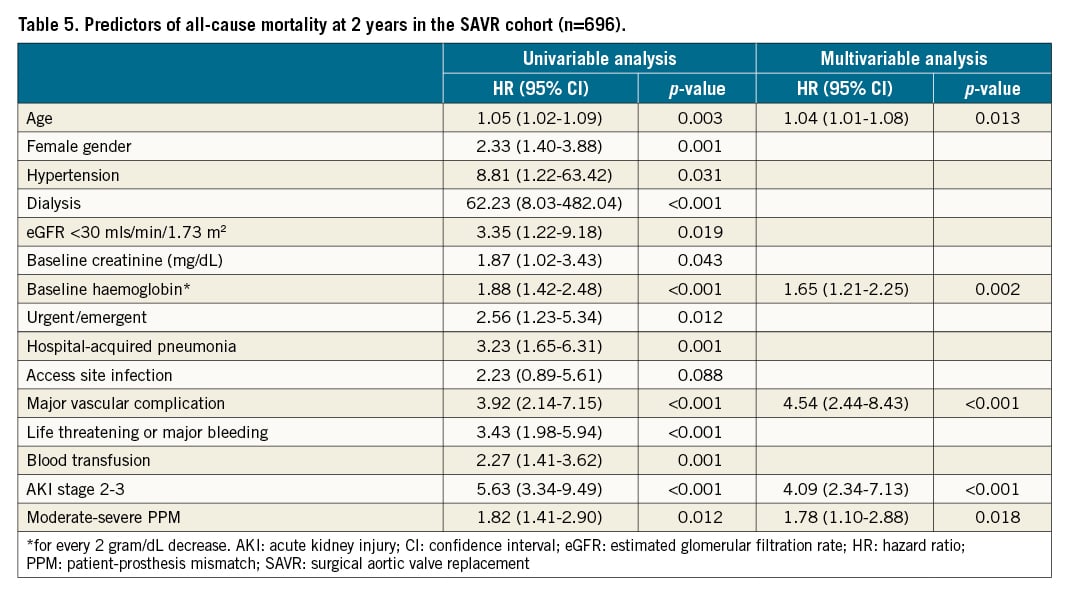
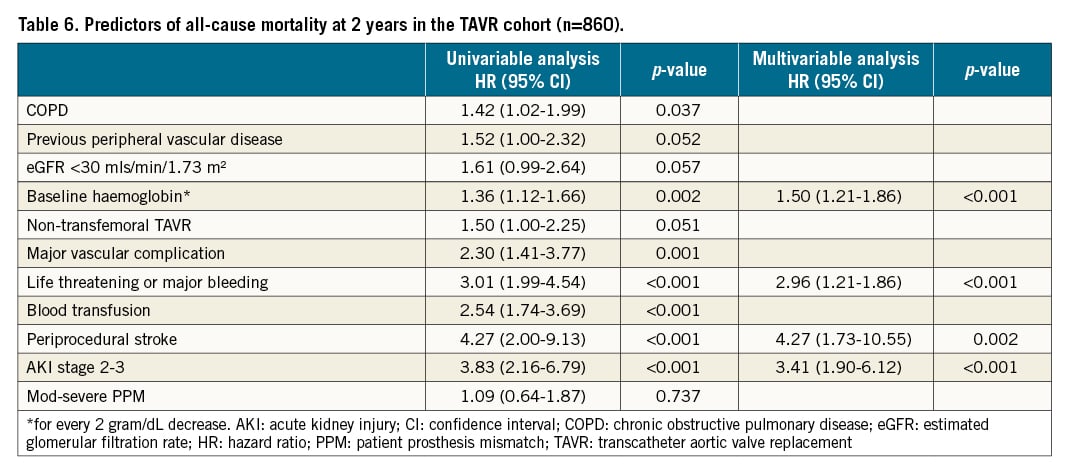
Predictors of all-cause 2-year mortality in the whole cohort of SAVR patients were: age, low baseline haemoglobin, and major vascular complications, AKI stage 2-3, and moderate to severe PPM (Table 5). Within the TAVR group, predictors of all-cause 2-year mortality were: low baseline haemoglobin, life-threatening or major bleeding, periprocedural stroke, and AKI stage 2-3 (Table 6). PPM was not a predictor of 2-year mortality in the TAVR group (p=0.737).
Patients undergoing TAVR or SAVR between 2014-2019
Considering only the propensity score-matched cohort of patients who underwent either TAVR or SAVR between 2014 and 2019, findings were similar to those for the whole cohort (Supplementary Table 3-Supplementary Table 5, Supplementary Figure 3, Supplementary Figure 4). Blood transfusions, AKI and moderate to severe PPM remained higher in the SAVR group. Interestingly, however, rates of permanent pacemaker implantation were not different between groups, in these more contemporary patients (11.1% versus 7.83%, for TAVR and SAVR, respectively; p=0.227). Mid-term outcomes, including all-cause mortality, CV mortality, all-cause readmission and CV readmission were not different between groups (log-rank p>0.05 for all comparisons).
Discussion
Our study compares the in-hospital and mid-term outcomes in MO patients with symptomatic severe AS undergoing either TAVR or SAVR. The main findings are as follows: 1) MO TAVR patients have lower periprocedural complications, except for a higher rate of permanent pacemaker implantation; 2) higher residual mean gradient and moderate to severe PPM were more frequently found following SAVR, and SAVR was an independent predictor of moderate to severe PPM; 3) TAVR patients have more residual moderate to severe aortic regurgitation than SAVR patients; 4) no difference in mid-term outcomes were seen between the TAVR and SAVR groups on propensity score matching, except for an increased all-cause readmissions at 2 years in SAVR patients in the matched, adjusted analysis; and 5) moderate to severe PPM was associated with 2-year all-cause mortality in the SAVR group but not in the TAVR group.
Outcomes in obese patients undergoing TAVR or SAVR have previously been heavily debated. Previous studies in obese versus normal weight patients undergoing SAVR, with and without coronary revascularisation, have shown conflicting results regarding in-hospital and 30-day mortality72526. In the context of TAVR, our research group has previously shown no differences regarding in-hospital or 30-day mortality for MO versus normal weight patients9. Studies comparing TAVR versus SAVR in this group are few. An analysis of the Nationwide Inpatient Sample database (NIS) in the United States showed no differences regarding in-hospital mortality between TAVR and SAVR patients with BMI ≥30kg/m2, or when the population was restricted to patients with BMI ≥40 kg/m2, although perioperative complications were more common in the SAVR group13. Our results are reflective of these findings. The less invasive nature of TAVR, particularly when performed via the femoral route (>85% in this study), likely explains the reduced in-hospital complications and significantly shorter in-hospital stay in the TAVR MO cohort. The ability to circumvent these periprocedural complications may suggest that TAVR in this particular population could be considered a more appropriate option for the treatment of symptomatic severe AS. It should be noted that while in-hospital mortality was not significantly different between groups, there was a trend towards greater in-hospital mortality in the SAVR group, with an absolute difference of 2.2%. Lack of statistical significance relating to this variable may reflect a lack of power in our study, and further larger studies should aim to definitively answer this question.
Conduction disorders and the need for permanent pacemaker implantation continue to be higher following TAVR, compared to SAVR. Consistent with previous studies, TAVR patients had a 2½-fold increased requirement for permanent pacemaker implantation than the SAVR cohort8. More recently, changes to implantation techniques, particularly with self-expanding TAVR valves, have shown promise in reducing pacemaker implantation rates2728. This is reflected in our analysis of patients who underwent TAVR and SAVR from 2014 to 2019. No differences were found between groups regarding permanent pacemaker requirement, and this is most likely due to current TAVR implantation techniques aimed at reducing pacemaker requirement. This represents an important finding given that pacemaker implantation is often considered a significant drawback of TAVR procedures.
Prosthesis-patient mismatch is known to occur in both TAVR and SAVR. In obese patients, the effect of PPM on outcomes was noted to be attenuated after SAVR and led to the use of BMI-adjusted cut-offs2129, which have now also been widely adopted in the assessment of PPM for patients undergoing TAVR procedures101922. Given that increased BMI and obesity is a known risk factor30, increased PPM may be expected in our cohort. However, rates in this study were similar or lower than previously reported in other TAVR and SAVR trials103031. This may be explained by the use of predicted, rather than measured, EOA across both TAVR and SAVR groups, which has been shown to result in lower rates of PPM1022 and to correlate more closely with transvalvular mean gradient22. Nonetheless, our study demonstrated higher residual mean gradients and a 4-fold higher rate of moderate to severe PPM in those who underwent SAVR, consistent with previous studies comparing SAVR to both balloon- and self-expanding TAVR valves103132. Smaller valve sizes were implanted more frequently in the SAVR group and were significantly associated with PPM in our study, as in other studies31. Implantation of larger-sized prostheses in TAVR patients compared to SAVR patients may be explained by the use of computed tomography (CT)-based sizing for TAVR valves, which is now widely accepted as standard practice33. A CT subanalysis of the SURTAVI trial demonstrated this by dividing patients by indexed annulus size into small, medium and large annuli. Across these subgroups, the size of implanted TAVR valves increased accordingly, while the size of implanted SAVR valves remained unchanged32. This suggests that the accurate annulus sizing, as provided by CT, used in TAVR populations most likely contributes to the choice of larger valve sizes and lower PPM in this group.
PPM is not a benign entity, and in our study moderate to severe PPM was associated with an increased risk of all-cause mortality at 2 years in the SAVR group, but not in the TAVR group. Analysis of the PARTNER 1 and 2 trials have similarly shown an association between PPM and mortality in the SAVR, but not the TAVR, group1031, although only severe PPM using predicted EOA values were associated with poorer outcomes in the analysis of PARTNER 2 (HR 3.30, 95% CI: 1.76-6.21; p<0.0001 for all-cause mortality and rehospitalisation)10. Likewise, in a large meta-analysis of TAVR and SAVR trials, no association with mortality was seen in patients with PPM following TAVR implantation34. Furthermore, PPM has been associated with structural valve deterioration in surgical bioprostheses35, and more recently been linked to subclinical valve thrombosis in TAVR, which may be a contributing factor to valve degeneration36. These findings highlight the need to avoid PPM, if possible, when performing TAVR or SAVR. Our findings, consistent with other studies of reduced rates of PPM following TAVR, may suggest an advantage of TAVR over SAVR in MO patients who are at particular risk of this complication.
Despite differences in periprocedural complications, mid-term outcomes were similar in both the propensity score-matched and adjusted analysis, except for all-cause rehospitalisation, which after adjustment for eGFR, LVEF and risk score, was more common in the SAVR group. Our matched cohort consisted of predominantly low-risk (~75%) patients, due to low numbers of high-risk patients in the surgical cohort overall. These results are important in the current TAVR era, where there is no direct randomised comparison between SAVR and TAVR in MO patients, and TAVR is expanding to younger and lower-risk populations.
Predictors of 2-year mortality were analysed separately for the TAVR and SAVR cohorts. Stage 2-3 AKI was a significant predictor of 2-year mortality across both groups. The rate of AKI across both groups was higher than in other studies of low- to intermediate-risk patients. This may reflect the comorbidity burden of our cohort, with high rates of diabetes, hypertension and underlying chronic kidney disease. Nonetheless, while stage 2-3 AKI predicted 2-year all-cause mortality in both groups, its significantly higher incidence in the SAVR group is worth considering when choosing between SAVR and TAVR in MO patients. Readmission rates were high with the majority being non-cardiac in nature, consistent with previous literature37. Further studies should centre on initiatives aimed at reducing readmission rates.
Limitations
A number of limitations must be recognised. Firstly, this is a retrospective analysis of prospectively collected data and, as such, has limitations inherent to this observational design. Although propensity score matching aims to eliminate significant differences between groups, the presence of unidentified confounding factors cannot be excluded. Long-term echo data regarding valve performance were not available, so an assessment of structural valve deterioration or haemodynami dysfunction cannot be reliably assessed. Therefore, our findings should be considered as hypothesis generating and require confirmation in future studies. However, the most clinically important CV comorbidities and potential confounders were included in the propensity score analysis, and matching resulted in well-balanced groups. Propensity matching, however, results in a reduced number of patients being included, which may limit the power to detect differences between groups. Lastly, median follow-up was close to 3 years, therefore, longer follow-up is necessary to determine potential differences in valve durability and survival across both groups.
Conclusion
In our population of predominantly low-risk MO patients, TAVR resulted in less periprocedural complications than those undergoing SAVR, however, rates of new permanent pacemaker implantation and significant aortic regurgitation were higher. Moderate to severe PPM was more common in the SAVR group and was associated with 2-year all-cause mortality in this group. Both therapeutic options resulted in similar mid-term outcomes, including all-cause mortality, CV mortality, all-cause readmission and CV readmission. However, after adjustment, all-cause readmissions were more common among SAVR patients. Our study suggests that TAVR in MO patients offers advantages over SAVR, in terms of periprocedural morbidity, with similar mid-term outcomes.
Impact on daily practice
Morbidly obese patients have been largely underrepresented in clinical trials to date comparing TAVR and SAVR. This study demonstrates that in a predominantly low-risk group of patients, TAVR results in less periprocedural morbidity with equivalent mid-term outcomes to SAVR. Furthermore, patient-prosthesis mismatch was more common in SAVR patients and has a significant impact on mid-term mortality. Therefore, TAVR can circumvent many of the complications associated with SAVR in MO patients and should be considered in MO patients of low or moderate risk presenting with severe AS.
Conflict of interest statement
I. Amat-Santos is a proctor for Boston Scientific and Meril Life Sciences. S. Toggweiler reports institutional grant support from Boston Scientific, Fumedica, and Biosensors; financial fees from Boston Scientific, Medtronic, Biosensors, Medira, AtHeart Medical, Shockwave, Teleflex, and Veosource; and holds equity in Hi-D Imaging. F. Saia is a proctor for Edwards Lifesciences; and received consulting and lecture fees from Abbott Vascular, Edwards Lifesciences, and Medtronic. H. Ribeiro is a consultant for Edwards Lifesciences, Medtronic, and Boston Scientific. A. Regueiro is a proctor for Abbott. M. Barbanti is a consultant for Edwards Lifesciences, and Boston Scientific. G. Tirado-Conte holds a research-training contract “Rio Hortega” (CM21/00091) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III). N. Gonzalo has received speaker and consultancy fees from Abbot Vascular, Boston Scientific, and Philips. L. Nombela-Franco is a proctor for Abbott Vascular; and has received speaker honoraria from Edwards Lifesciences, and Boston Scientific. He also holds a research grant (INT19/00040) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III). S. Mohammadi is a a proctor for Edwards Lifesciences, and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

