Abstract
Aims: Transcatheter aortic valve implantation (TAVI) represents a less invasive treatment option for elderly patients. Therefore, we aimed to determine the impact of frailty measured by the Katz Index of activities of daily living (ADL) on short- and long-term mortality after TAVI.
Methods and results: Our study included 300 consecutive patients (mean age, 82±5 years) who had undergone TAVI at our institution (158 transapical, 142 transfemoral procedures). At baseline, 144 patients were impaired in at least one ADL and therefore defined as frail (Katz Index <6). Regarding in-hospital outcome, all serious complications except for stage 3 acute kidney injury were equally distributed in both groups, but early mortality was significantly higher in frail persons (5.5% vs. 1.3%, p=0.04 for immediate procedural mortality; 17% vs. 5.8%, p=0.002 for 30-day mortality; and 23% vs. 6.4%, p<0.0001 for procedural mortality). The risk-score-based 30-day mortality estimates (29% vs. 24% for log. EuroSCORE I, 9.5% vs. 7.5% for EuroSCORE II, and 8.8% vs. 5.9% for STS score) reflected neither the observed 30-day mortality in both groups nor the threefold risk elevation in frail patients. In contrast, the Katz Index <6 was identified as a significant independent predictor of long-term all-cause mortality by multivariate analysis (HR 2.67 [95% CI: 1.7-4.3], p<0.0001). During follow-up (median observation period 537 days) 56% of frail vs. 24% of non-frail patients died.
Conclusions: Frailty status measured by the Katz Index represents a powerful predictor of adverse early and late outcome after TAVI, whereas commonly used risk scores lack calibration and discrimination in a TAVI-specific patient cohort. Therefore, we propose the incorporation of this simple and reproducible measure into pre-TAVI risk assessment.
Introduction
Aortic stenosis (AS) of degenerative origin has become the most frequent type of valvular heart disease in Europe and North America1,2, and its prevalence increases substantially with ageing, rising to 2.8% in individuals above 75 years of age2. Taking into account the demographic shift to an older population, the burden of degenerative aortic valve disease is expected to increase further in the future. Due to the availability of transcatheter aortic valve implantation (TAVI), nowadays a less invasive treatment option can be offered to patients who are considered to be too high-risk for valve surgery and who, ten years ago, would therefore only have received medical treatment. This, however, challenges our health systems with increasing numbers of aged and multimorbid patients referred for treatment of AS. Age and associated comorbidities determine operative risk and life expectancy which both have important implications on decision making. According to current guidelines3, TAVI is recommended in patients with severe symptomatic AS who are considered unsuitable or at high risk for conventional surgery, and the risk assessment should mostly rely on the Heart Team’s clinical judgement in addition to a combination of surgical risk scores. Nevertheless, “eligible patients should have a life expectancy of more than 1 year and should also be likely to gain improvement in their quality of life, taking into account their comorbidities”3. Conflicting with this recommendation, the observed mid- and long-term mortality after TAVI is considerable, with reported one- and two-year mortality rates of 24% and 33% in high-risk patients4,5 and 31% and 43% in inoperable individuals6, respectively. So, which tools do we have to appraise life expectancy and potential benefit from TAVI in elderly patients with severe symptomatic AS in order to identify appropriate candidates for this intervention? The surgical risk scores commonly used to estimate perioperative morbidity and mortality provide relatively good discrimination, enabling a gross estimation of risk category. However, in valvular heart disease they do not provide a reliable estimate of the exact operative risk (and still less of long-term survival) in an individual patient due to a significant lack of calibration7,8.
Frailty is a very prevalent condition among patients undergoing TAVI today and has been associated with poorer outcomes after cardiac surgery9-11. However, it is not incorporated in current risk assessment, although a growing body of evidence suggests that its presence also exhibits a relevant impact on outcome after transcatheter aortic valve implantation. To shed more light on this subject, we aimed to determine the impact of frailty on short- and long-term morbidity and mortality in a single-centre cohort of patients undergoing TAVI procedures. Therefore, we collected data concerning the Katz Index of activities of daily living (ADL), a widely accepted measure of dependency in elderly individuals12.
Methods
PATIENT POPULATION AND STUDY DESIGN
This observational study, completely independent from the industry, includes the first 300 consecutive patients undergoing TAVI at our institution between August 2008 and February 2012. Patient and approach selection for TAVI was at the discretion of the “Heart Team” and was carried out according to current recommendations3. All potential patients underwent a systematic process of clinical evaluation, echocardiographic assessment, and coronary and aorto-iliofemoral angiography. The access route was chosen mainly in consideration of the size, tortuosity, calcification and atheroma of the aorto-iliofemoral arteries. One hundred and fifty-eight (158) patients (53%) were treated via the transapical and 142 (47%) via the transfemoral approach. In the latter group, the Medtronic CoreValve® (Medtronic, Minneapolis, MN, USA) was used in 28 cases, and in all other procedures the Edwards SAPIEN valve (Edwards Lifesciences Inc., Irvine, CA, USA) was implanted.
At baseline, patient comorbidities (defined with the EuroSCORE definitions) were recorded, and deficiencies in the Katz Index of ADL (independence in feeding, bathing, dressing, transferring, toileting, and urinary continence) as well as impairment in ambulation (inability to walk independently, use of walking sticks permitted) or cognition (previous diagnosis of dementia or signs of temporal, topographical or personal disorientation on admission) were routinely assessed by trained nurse practitioners. Patients with any deficiency in the Katz Index were defined as frail for the purpose of this study. At discharge, periprocedural complications and mortality rates were evaluated according to VARC-2 definitions13, and echocardiography was repeated.
All 300 patients were followed by regular telephone contact which was last performed around the turn of the years 2012/2013. Thus, at least one-year follow-up was obtained for all patients. NYHA status and further hospitalisations were investigated using a standardised questionnaire, and medical documents were acquired to establish the causes of death and re-hospitalisations. To assess patients’ subjective benefit of TAVI, we furthermore asked how quality of life had changed as a consequence of the intervention, with the possible options “significantly improved”, “slightly improved”, “unchanged”, and “worsened”.
The study was approved by the local ethics committee, and written informed consent was obtained from all patients.
STUDY ENDPOINTS
Recently, an updated consensus report from the Valve Academic Research Consortium (VARC-2) proposed standardised endpoint definitions to enable comparison between TAVI trials13. VARC-2 definitions which are described in detail in the consensus report were adopted for reporting of periprocedural complications in the present study. Concerning follow-up, all-cause mortality was defined as the primary clinical endpoint according to VARC-2 proposals. Mortality rates were reported for immediate procedural mortality (within 72 hours after TAVI), at 30 days, for procedural mortality (defined as death within 30 days or during index procedure hospitalisation, if the postoperative length of stay was longer than 30 days), at six months, one year, and two years.
Additionally, the VARC-defined composite early (30 days) safety endpoint consisting of all-cause mortality, all strokes, life-threatening bleeding, acute kidney injury stage 2 and 3, coronary artery obstruction requiring intervention, major vascular complications and valve-related dysfunction requiring repeat procedure was reported.
STATISTICAL ANALYSIS
Statistical analysis was performed with GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA, USA) and with the statistical computing software R version 2.15.1 (http://www.r-project.org). Continuous variables were compared using the Mann-Whitney U test (absence of normality distribution). Categorical variables are presented as absolute numbers and percentage and were compared by Pearson’s chi-square test. A value of p<0.05 was considered statistically significant.
Survival analysis was performed on time-to-event data (i.e., time to death of any cause) using the R package “survival”. Survival data were visualised by Kaplan-Meier plots, and significance was calculated by the log-rank test (for two-group comparisons). For multivariate models, the Cox proportional hazards model was used. Survival analyses were performed for baseline characteristics, risk scores and Katz Index. Risk stratifiers that were found to be significant in univariate analyses were included in multivariate analyses. Due to major redundancies among the different frailty measures, only the scale with the highest hazard ratio for mortality prediction was chosen for multivariate analysis. The parameters “age” and “gender” were also inserted into this model, because they differed significantly in frail and non-frail individuals.
Results
BASELINE AND PROCEDURAL CHARACTERISTICS
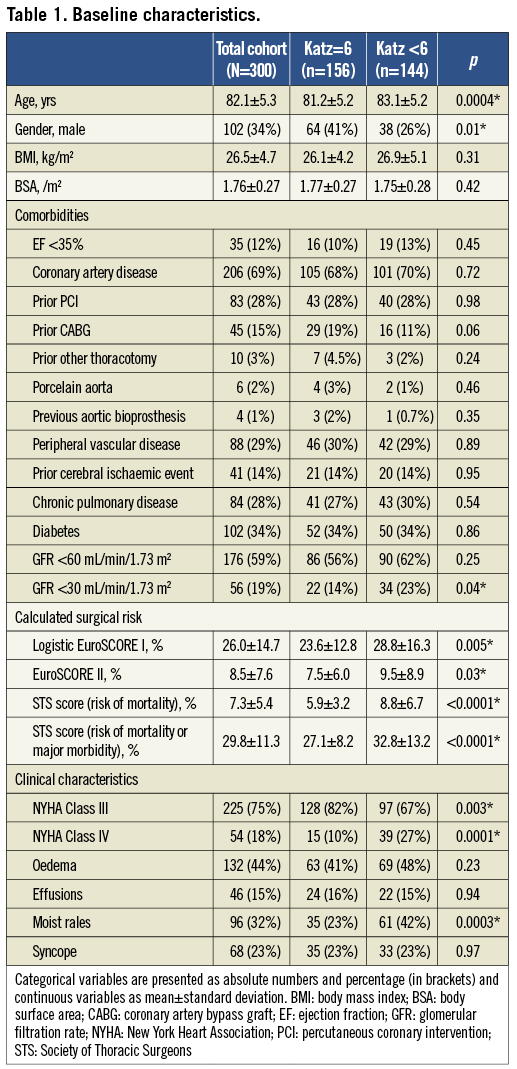
The patient cohort was characterised by advanced age (mean 82.1±5.3 years) and a significant burden of comorbidities leading to a high surgical risk expressed by high measures for common surgical risk scores (logistic EuroSCORE I 26.0±14.7%, EuroSCORE II 8.5±7.6%, STS score 7.3±5.4%) (Table 1). Coronary artery disease was present in 69%, renal insufficiency in 59% (GFR <30 mL/min/1.73 m2 in 19%), diabetes mellitus in 34%, chronic pulmonary disease in 28%, a prior cerebral ischaemic event in 14%, and previous cardiac surgery had been performed in 18%. Furthermore, our patients showed severe symptoms of congestive heart failure, with 93% presenting in New York Heart Association (NYHA) Classes III and IV. Of note, only one third of patients were male. One hundred and forty-four persons (48%) were impaired in at least one ADL and therefore defined as frail (Katz Index <6), whereas 156 individuals (52%) were completely independent in ADL (Katz Index =6). Furthermore, 92 patients (31%) exhibited impairment in ambulation and 25 patients (8%) cognitive impairment.
Compared to independent individuals, patients with any impairment in ADL were on average two years older (p=0.0004), more likely to be female (p=0.01), and exhibited a higher prevalence of renal insufficiency with GFR <30 mL/min/1.73 m2 at baseline (p=0.04) (Table 1). Furthermore, they presented in more advanced stages of congestive heart failure (NYHA Class IV in 27% vs. 10% at baseline, p=0.0001), and clinical signs of heart failure were significantly more frequent. However, baseline echocardiographic parameters (ejection fraction, transvalvular gradients, aortic valve area, pulmonary artery systolic pressure, prevalence and degree of aortic regurgitation) did not differ. Concerning procedural characteristics, no differences in total procedure time (p=0.52), fluoroscopy time (p=0.92), or volume of contrast medium (p=0.85) were observed between both groups.
IN-HOSPITAL OUTCOME
Incidences of specific complications (definitions adopted from the VARC-2 consensus document13), mortality rates and causes of death are demonstrated in Table 2 and Table 3 for the total cohort as well as for frail and non-frail individuals.
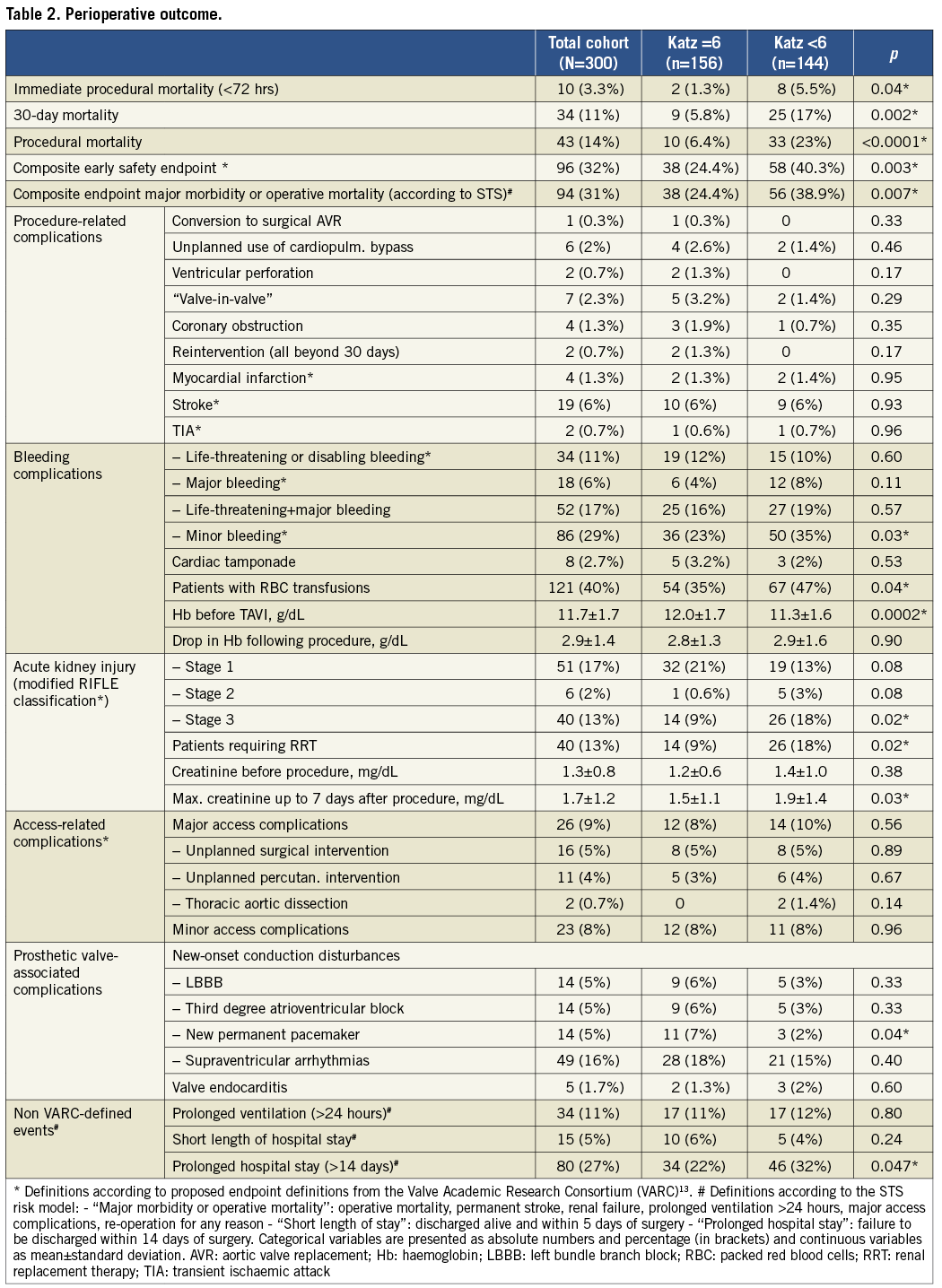
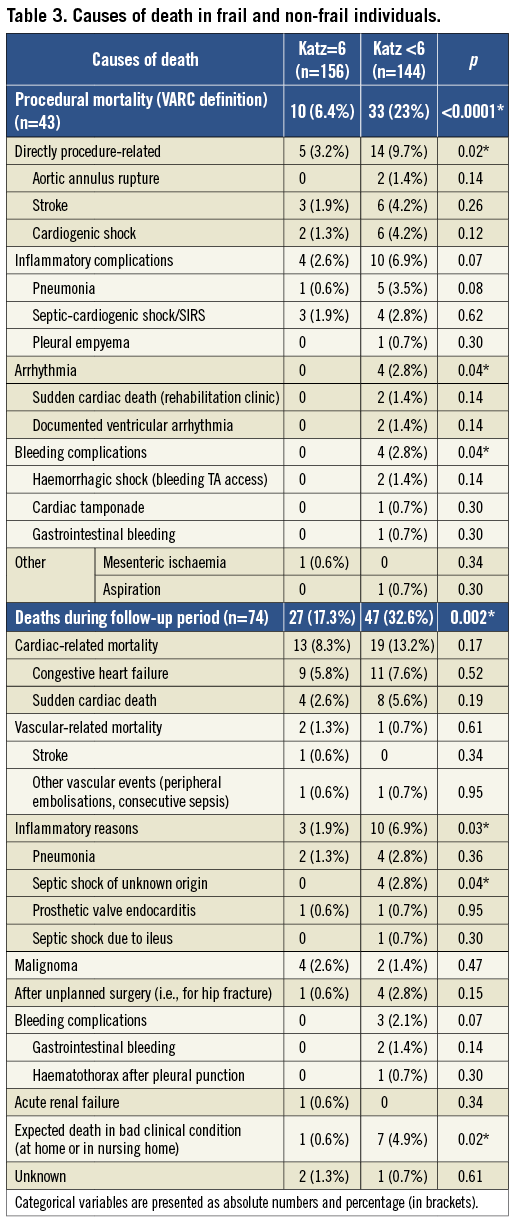
The only complication with significantly different incidence in both groups was stage 3 acute kidney injury, which is not surprising bearing in mind that patients with Katz Index <6 had significantly worse renal function at baseline. All other serious complications occurred with equal distribution in both groups (p=0.93 for stroke, p=0.95 for myocardial infarction, p=0.35 for coronary obstruction, p=0.60 for life-threatening bleeding, p=0.56 for major access complications). However, mortality rates at each evaluated point in time were significantly higher in patients with any impairment in ADL compared to independent persons (5.5% vs. 1.3% immediate procedural mortality, 17% vs. 5.8% 30-day mortality, and 23% vs. 6.4% procedural mortality). This fact could be explained by the finding that frail patients were at a significantly higher mortality risk when serious complications occurred. For example, no independent person died from bleeding or access complications, but in impaired patients four out of 15 patients (27%) died from bleeding (p=0.02 compared to non-frail) and five out of 14 persons (36%) from major access complications (p=0.02). Similarly, major strokes were observed in 10 independent patients and caused three subsequent deaths (30%), whereas six out of nine (66%) patients with Katz Index <6 and postoperative stroke died (p=0.1). Additionally, pneumonia as cause of death was more frequent in individuals with Katz Index <6 (five patients vs. one patient, p=0.08).
Regarding risk-score-based mortality estimates, all calculated values were significantly higher in frail patients (29% vs. 24% for log. EuroSCORE I, 9.5% vs. 7.5% for EuroSCORE II, and 8.8% vs. 5.9% for STS score). However, these estimates reflected neither the observed 30-day mortality in both groups (17% vs. 5.8%) nor the threefold risk elevation in frail patients (Figure 1). The VARC-defined composite early safety endpoint was met significantly more frequently in frail patients (40.3% vs. 24.4%, p=0.003), which was mainly driven by 30-day mortality and the incidence of acute kidney injury. Furthermore, patients with any impairment in ADL were at significantly higher risk of prolonged hospital stays >14 days (32% vs. 22%, p=0.047; median length of prolonged stays, 23 days). Additionally, temporary or permanent dependence on institutional nursing at time of discharge after TAVI was significantly more frequent in patients with baseline Katz Index <6 compared to independent persons (59/111 vs. 13/146 patients discharged alive, p<0.0001).
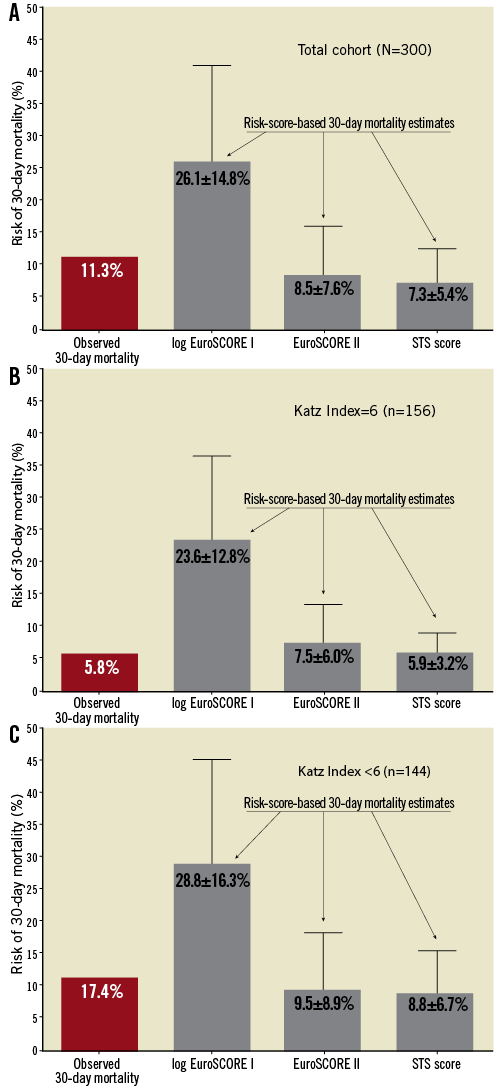
Figure 1. Comparison of risk-score-based 30-day mortality estimates with observed 30-day mortality in the total cohort (A) as well as in non-frail (B; Katz Index =6) and frail (C; Katz Index <6) persons reveals lack of score calibration and discrimination in this TAVI-specific patient cohort. For risk scores, mean and standard deviations of mortality estimates are indicated.
ANALYSIS OF ALL-CAUSE MORTALITY DURING FOLLOW-UP
The follow-up for survival status and incidence of MACCE was 100% complete. Median observation period (time to last contact or death) was 537 days (range: 0-1,491 days). Overall survival was 89% at 30 days, 79% at six months, 72% at 12 months, and 63% at two years (see also survival curve for the total cohort in Figure 2A). Altogether, 117 patients (39%) had died at the time of last follow-up (Table 3).
Survival analyses were performed to test the potential of different baseline parameters, risk scores and frailty status to predict mortality. Univariate analyses identified the transapical access, NYHA functional Class IV at baseline, reduced LV ejection fraction (<55%), pulmonary artery systolic pressure ≥44.5 mmHg, glomerular filtration rate (GFR) <30 mL/min/1.73 m2 and <60 mL/min/1.73 m2, diabetes mellitus, preoperative anaemia (haemoglobin <11.5 g/dL), all three surgical risk scores, and the frailty status (measured either by Katz Index, Barthel Index, modified Rankin Scale or according to Lee et al10) as significant predictors of mortality (Table 4). Importantly, other established risk factors for elevated mortality in conventional surgery, such as age, female gender, previous cardiac surgery, peripheral vascular disease and chronic lung disease, did not predict mortality in our TAVI patient cohort.
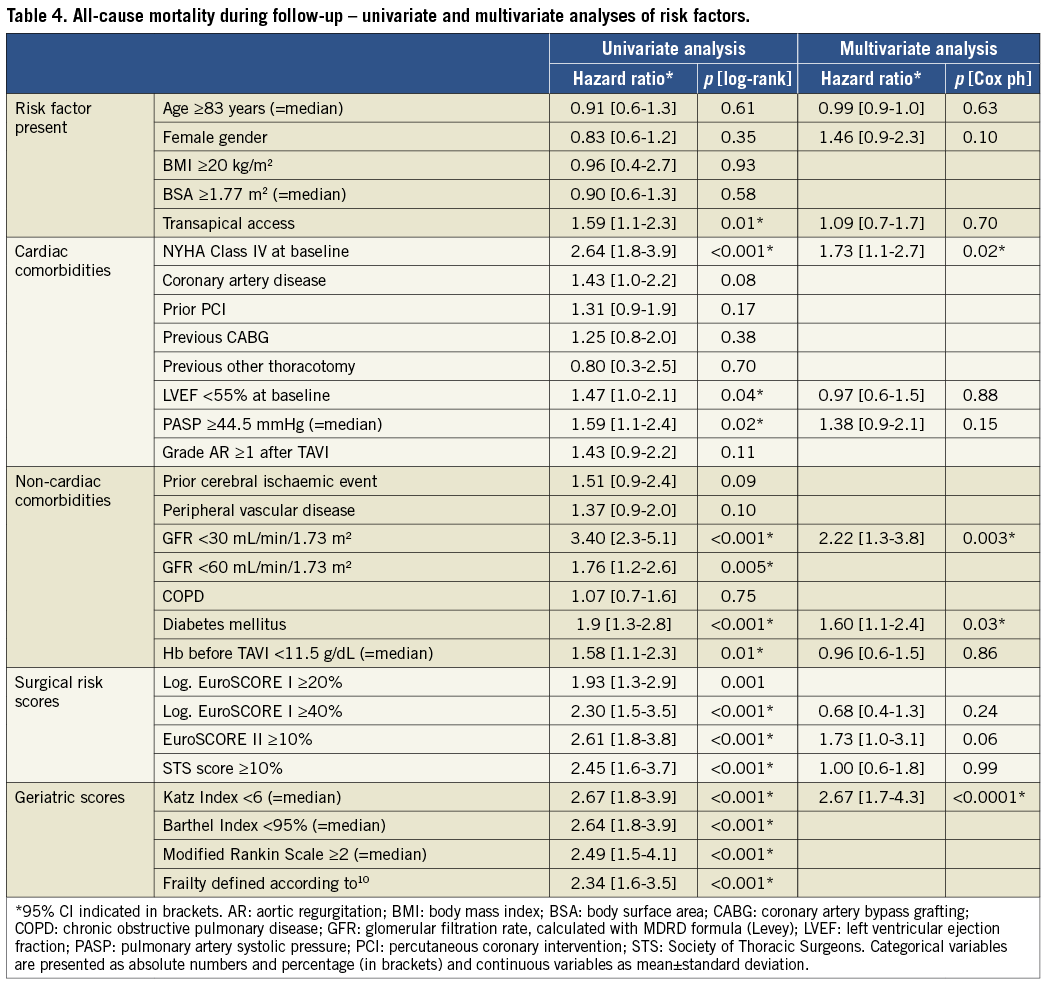
Risk factors which were found to be significant in univariate analyses were tested in multivariate analyses using a Cox proportional hazards model. The parameters “age” and “gender” were also inserted into this model, because they differed significantly in frail and non-frail individuals (Table 1). Due to major redundancies among the different frailty measures, only the Katz Index (which represented the scale with the highest hazard ratio for mortality prediction) was chosen for multivariate analysis. In this model, only the four parameters: NYHA Class IV at baseline, GFR <30 mL/min/1.73 m2, diabetes mellitus, and Katz Index <6 could be identified as significant independent predictors of all-cause mortality (Table 4), and the Katz Index <6 exhibited the highest hazard ratio for mortality prediction (2.67). Of note, no surgical risk score proved to have significant independent impact on overall survival beyond these risk factors. In conclusion, we identified frailty status measured by any impairment in ADL as the most important predictor of survival after TAVI in our patient cohort.
Survival comparison for dependent and independent individuals is displayed by Kaplan-Meier analysis in Figure 2A: 80/144 frail patients (56%, median survival 759 days) and 37/156 (24%) non-frail patients died during the observation period (p<0.0001). Furthermore, subgroup analysis according to the degree of frailty was performed with the two subgroups Katz Index 0-2 (severe disability) and Katz Index 3-5 (moderate disability). Figure 2B clearly illustrates that survival rates decrease constantly with increasing grade of disability: median survival was 998 days in moderately disabled and 279 days in severely disabled individuals (p=0.002). To clarify whether frailty status impacts mostly on early or late mortality, a landmark analysis was performed. Frailty defined as any deficiency in the Katz Index proved to be a significant predictor for 30-day mortality (p=0.003, HR 3.05, Figure 2C) as well as for follow-up mortality beyond 30 days (p<0.0001, HR 2.50; all deaths occurring between days 0 and 30 censored; Figure 2D). Interestingly, subgroup analysis revealed that patients with moderate disability (Katz Index 3-5) did not exhibit a significantly elevated 30-day mortality compared to independent persons (p=0.70) but a significantly lower one than severely disabled persons (Katz Index 0-2) (p=0.0008, HR 4.63). However, during long-term follow-up mortality rates in this moderately disabled cohort “caught up” and did not differ significantly from the severely disabled group’s any more (p=0.1, HR 1.56).
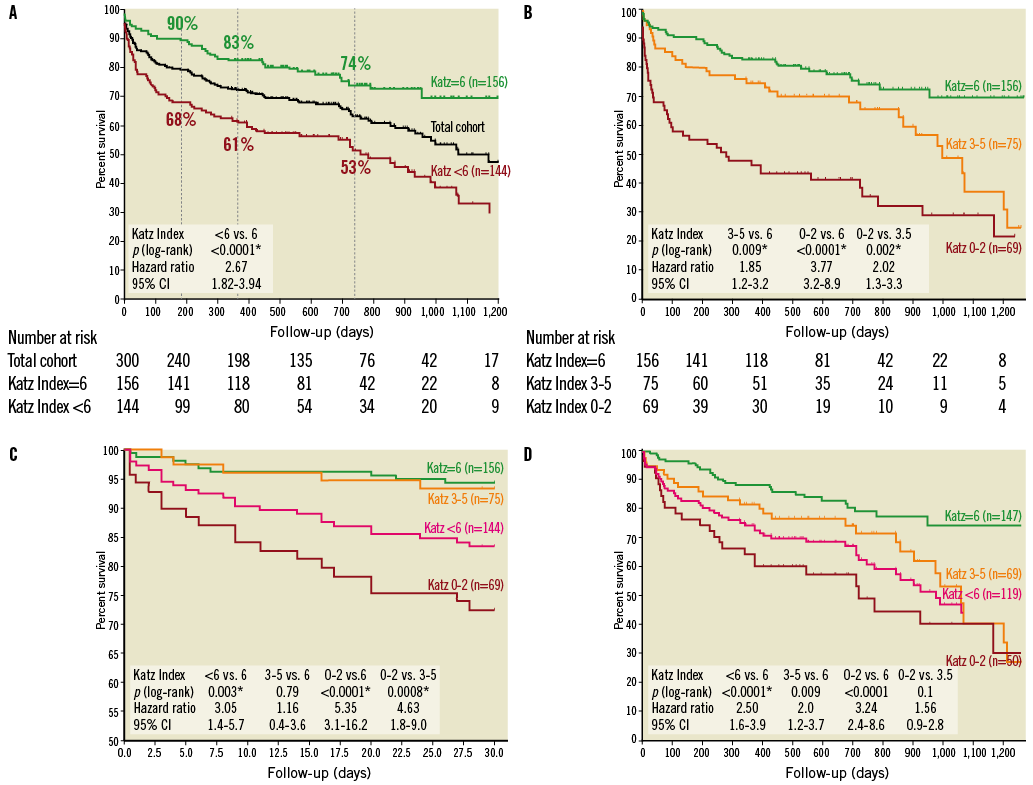
Figure 2. Survival depending on frailty status and its different stages. Kaplan-Meier curves demonstrating (A) the impact of frailty measured by any impairment in ADL at baseline (Katz Index <6) on overall survival (survival proportions at six months, one year and two years are indicated for frail and non-frail individuals), (B) the impact of the degree of frailty in the subgroups Katz Index 0-2 (severe disability) and Katz Index 3-5 (moderate disability) on overall survival, (C) a landmark analysis including only days 0-30 to determine the impact of frailty and its different stages on early mortality, and (D) a landmark analysis to determine the impact of frailty and its different stages on late mortality (all deaths between days 0 and 30 censored).
The excess mortality in individuals with baseline Katz Index <6 was especially pronounced in patients with additional GFR <30 mL/min/1.73 m2 who exhibited a median survival of only 86.5 days and a two-year mortality rate of 75%. Event-free survival was also significantly poorer in frail patients (median event-free survival of 395 days vs. 888 days in non-frail persons, p=0.002), but this finding was mainly driven by cases of death, whereas MACCE-related re-hospitalisations did not differ significantly between frail and non-frail persons.
Concerning subjective long-term benefit from TAVI, surviving patients with baseline Katz Index <6 were significantly less likely than independent persons to report significant improvement in their quality of life (48% vs. 64%, p=0.04), although an optimistic selection bias due to numerous deaths before time of last follow-up has to be postulated.
Discussion
In the present study, we were able to identify frailty status (defined as any impairment in ADL) as an independent predictor of adverse short- and long-term outcomes after TAVI procedures. Concerning the index hospitalisation, frail patients were at significantly higher risk of early mortality, of prolonged hospital stay (>14 days), and of dependence on institutional nursing at discharge. During follow-up, survival was significantly reduced in patients with any impairment in ADL, whereas persons presenting with Katz Index of 6 had excellent long-term survival rates. The frailty status was identified as the parameter with the highest impact on long-term survival after TAVI in our patient cohort. In contrast, all surgical scores failed to demonstrate significant independent impact on overall survival.
It is generally agreed that frailty is a geriatric syndrome of impaired resiliency to stressors that results from deterioration in multiple physiological systems. As a result, the frail person is at increased risk of death from minor external stresses. However, there is as yet no gold standard for defining and measuring frailty. In principle, two different concepts exist. An emerging consensus promotes a definition based on a specific phenotype of frailty which has been operationalised with five components: unintentional weight loss, weakness (measured by grip strength), self-reported fatigue (measured by questionnaire), slowness (measured by five-metre gait speed test), and diminished physical activity (measured by questionnaire)14. The proponents of this concept also demand a clear distinction between frailty and disability (defined as an impaired ability to carry out functional tasks, measured, i.e., by the Katz Index of ADL), and also comorbidity, which are three causally related but distinct clinical entities in their eyes15. The second approach questions the usefulness of this distinction and measures frailty in relation to the accumulation of deficits16. This more pragmatic approach aims at identifying people at higher risk of predefined adverse outcomes. This discussion on how to define frailty gives rise to the secondary question as to which tool could be appropriate for measuring frailty in daily clinical practice. A frailty assessment tool for our purpose should ideally enable risk stratification and identification of factors for potential modification. Furthermore, it should be characterised by simplicity, reproducibility, objectivity and applicability to the busy clinical setting. The so-called eyeball test is very simple to perform but clearly lacks objectivity and reproducibility. The available frailty scores are objective but relatively time-consuming. Physical performance assessments (such as grip strength) as surrogate markers are objective and reproducible, but sometimes do not point out clear areas for modification of frailty to the clinician.
Several publications deal with the impact of frailty on morbidity and mortality in elderly patients. It has been known for a long time that functional status (commonly evaluated by the ability to complete ADL and instrumental activities of daily living [IADL]) is a strong predictor of morbidity and mortality in the geriatric population. Inouye et al17 found that any ADL impairment was associated with a 1.9-fold increased risk of all-cause mortality at two years in a population of patients aged 70 years or older admitted to general medicine departments. Furthermore, it has been demonstrated that frailty and the onset of dependence in ADL are strongly associated18.
Lee et al10 explored the impact of frailty (in this study defined as any deficiency in the Katz Index of ADL, in ambulation, or with a previous diagnosis of dementia) on mortality in 3,826 patients undergoing cardiac surgery. Frailty proved to be an independent predictor of in-hospital mortality (HR 1.8) and reduced midterm survival (HR 1.5) during a median follow-up of 1.8 years. Consistent with Lee’s findings, Afilalo et al9 established five-metre gait speed as an incremental predictor of in-hospital mortality and major morbidity in 131 elderly patients (mean age 75 years) undergoing cardiac surgery, associated with a twofold to threefold increase in risk. A subsequent study by the same authors11 combined established cardiac surgery risk scores with different scales of frailty and disability to improve identification of elderly patients at increased risk of postoperative mortality or major morbidity.
There is also a growing body of evidence that measures of frailty and disability are able to improve risk stratification in the setting of severe AS and TAVI. The prevalence of frailty measured by five-metre gait speed in patients with severe AS was recently defined in a series of 102 persons who were screened for TAVI19. Slow gait speed was present in 63% of patients and was independently associated with disability (as measured by dependence in ADL). Also, the pre-interventional six-minute walking test distance proved to be an independent mortality predictor in 260 consecutive patients who underwent TAVI (HR 1.08 for each decrease in 10 metres, p=0.001)20. Two recent studies focus on the impact of frailty status on survival after TAVI. The first publication of Stortecky et al21 evaluated multidimensional geriatric assessment (cognition, nutrition, mobility, ADL, and frailty index) for risk stratification in 100 consecutive patients undergoing TAVI. All geriatric parameters were significantly associated with mortality and MACCE at 30 days and one year after TAVI. Green et al22 prospectively measured gait speed, grip strength, serum albumin, and ADL status in 159 subjects who underwent TAVI in order to derive a frailty score. Frailty status was not associated with increased periprocedural complications, but was associated with increased one-year mortality after TAVI. Additionally, Rodes-Cabau et al5 identified frailty (among other risk factors) as an independent predictor of two-year mortality in 339 patients treated with TAVI. However, frailty status was assessed by “eye-balling” and without objective measures in this study.
In our study, we were able to identify frailty (defined as any impairment in ADL) as an independent predictor of short- and long-term morbidity and mortality in the (so far) largest cohort of consecutive patients treated with TAVI. As already mentioned above, according to the predominant geriatric view, this criterion does not denote frailty, but defines disability. However, some components of the frailty phenotype definition14 do not seem to provide accurate discrimination in this specific patient cohort. For example, low BMI or BSA did not predict adverse outcome in our patients, which is potentially attributable to the well-known fact that the use of weight loss to capture age-related loss of muscle mass fails to capture the frailty phenotype in obese older adults16. Additionally, recent studies have already demonstrated a strong association between accepted measures of frailty (i.e., gait speed) and disability (measured by dependence in ADL)18,19. Thus, we decided to use the term “frailty” for our purpose, because the term “disability” is not common in the TAVI-related literature and because the distinction between frailty and disability seemed to us to be rather a question of nomenclature than of clinical impact. Recently, Lee et al10 also proposed a definition of frailty as any deficiency in the Katz Index of ADL, in ambulation, or with a previous diagnosis of dementia. However, in our own patient cohort this frailty definition led to a lower discriminatory power for risk stratification (HR 2.34, p<0.0001) compared to a frailty definition based on the Katz Index of ADL alone (HR 2.67, p<0.0001). Finally, we therefore chose this very simple definition of frailty status which fulfils the criteria of simplicity, reproducibility, objectivity and applicability to the busy clinical setting, but which could nevertheless provide a powerful risk stratification in our patient cohort. Gait speed, the best validated parameter so far concerning frailty-associated mortality risk following cardiac procedures, and six-minute walking distance were not measured in our study which has to be acknowledged as a study limitation. Nevertheless, our present findings do point to a clear potential area for modification, namely the use of physiotherapy and ergotherapy to improve functional status prior to TAVI. Further studies will have to clarify whether such preventive steps are able to improve long-term outcome after TAVI. Moreover, our results may provide arguments in favour of performing the procedure earlier in patients’ history and even of screening elderly patients for presence of severe AS to prevent pre-interventional loss of functional capacities. As soon as a patient has crossed this threshold and modifying measures are futile, survival after TAVI is very limited, and no TAVI trial has as yet demonstrated a survival benefit in this specific patient population. Ben-Dor et al23 followed a cohort of 274 inoperable patients (mean log. EuroSCORE 42.4%, STS score 12.8%) who were screened but not eligible for TAVI trials and treated with medical therapy plus balloon valvuloplasty: overall survival was 68% at six months, 60% at one year and 47% at two years - which is absolutely concurrent with survival proportions after TAVI in our own (nominally at lower operative risk) frail patient subcohort (overall survival 68% at six months, 61% at one year and 53% at two years). As a consequence of these data, frail patients should be informed that the outcome of TAVI is uncertain in their situation.
Study limitations
The present investigation represents a single-centre experience with a relatively small sample size (n=300) which could be regarded as a limitation of the study. However, the complete analysis of consecutive patients without any exclusion and with a 100% complete follow-up (which is never realised in registries) is a forte of our data.
Second, 30-day mortality was somewhat higher in our series (11.3%) if compared to recent similar registry data5,24-27 reporting mortality rates ranging from 5.9%26 to 10.6%5, which could not be attributed to an early learning curve. This fact was driven by a higher early mortality in our transapical cohort (16.5%) compared to registry publications (7.7%26 to 11.3%5), whereas 30-day mortality of our transfemoral patients (5.6%) compared favourably to other recent reports (5.1%26 to 9.9%5). We suggest that the reason might be the worse functional status of our patients who exhibited the highest prevalence of NYHA status ≥3 (93%) when compared to other registries5,24-27.
Conclusions
Frailty status measured by any impairment in ADL (Katz Index <6) represents a powerful tool for pre-TAVI risk assessment. However, current TAVI guidelines3 only mention surgical risk scores for the assessment of preoperative risk (which do not incorporate any measures of frailty). For our TAVI-specific patient cohort, we were able to demonstrate a significant lack of calibration of commonly used risk scores with, i.e., overestimation of 30-day mortality risk of up to fourfold by log. EuroSCORE I (in non-frail patients) or underestimation of nearly 100% by STS score (in frail persons) (Figure 2). Therefore, the utility of such risk score estimates should be questioned in the setting of TAVI. In contrast, baseline assessment of frailty status identified individuals without impairment in ADL as a subgroup with excellent long-term survival and particular benefit from TAVI, whereas frail patients exhibited poor long-term survival. This issue is of utmost importance in the context of an ageing population, growing healthcare expenditures and consecutive need for treatment allocation of appropriate patients who are able to gain benefit from this costly intervention. In summary, we therefore propose the incorporation of the Katz Index of ADL into pre-TAVI risk assessment. Moreover, we hypothesise that means to eliminate or prevent impairment in ADL may improve outcome after TAVI.
| Impact on daily practice Frail patients (defined by the Katz Index <6) are at high risk of adverse early and late outcome after TAVI and should therefore be informed that a treatment success is uncertain in their situation. Moreover, our results may provide arguments in favour of performing the procedure as early as possible in the patients’ history to prevent pre-interventional loss of functional capacities. Further studies will have to clarify whether preventive steps like physiotherapy are able to improve long-term outcome after TAVI in frail individuals. |
Conflict of interest statement
M. Puls, R. Seipelt and W. Schillinger received compensation for travel expenses, and R. Seipelt and W. Schillinger also received proctor fees from Edwards Lifesciences. The other authors have no conflicts of interest to declare.

